May 2008
·
141 Reads
·
67 Citations
Molecular Vision
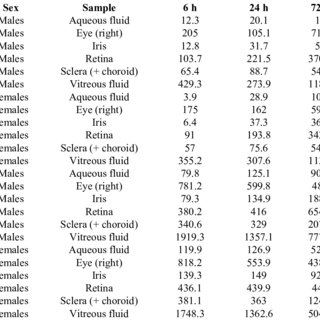
The primary objective of these investigations was to determine the ocular biodistribution of bevasiranib, a small interfering RNA (siRNA) targeting vascular endothelial growth factor A (VEGF-A), following a single intravitreal injection to rabbit eyes. A tissue distribution and pharmacokinetic study was conducted with (3)H-bevasiranib prepared in balanced-salt solution (BSS). Single doses of either 0.5 mg/eye or 2.0 mg/eye of (3)H-bevasiranib were given by intravitreal injection to Dutch-Belted rabbits (both eyes were treated). Subgroups of rabbits were serially-sacrificed at various times up to 7 days following dosing for collection of tissue samples. The right eye of each rabbit was collected whole, and the left eye was dissected to isolate five ocular tissues. All samples were analyzed by liquid scintillation counting to determine the concentrations of bevasiranib equivalents. An ocular disposition study was also performed with non-radiolabeled bevasiranib, which was administered to Dutch-Belted rabbit eyes via intravitreal injection at a dose of 2.0 mg/eye. Twenty-four hours post-dose, the eyes were enucleated and dissected into eight individual ocular structures that were analyzed for intact bevasiranib using a locked nuleic acid (LNA) noncompetitive hybridization-ligation enzyme-linked immunosorbent assay. Following intravitreal injection of 0.5 mg or 2.0 mg radiolabeled bevasiranib to Dutch-Belted rabbits, bevasiranib was detected in the vitreous, iris, retina, retinal pigment epithelium (RPE), and sclera (+choroid). As expected, the highest concentrations were found in the vitreous, and vitreous levels steadily decreased over time, while concentrations of radioactivity in the other ocular tissues increased to maximum values between 24 h and 72 h after dosing. Of these tissues, the highest concentration of radioactivity was detected in the retina. The LNA assay further confirmed the presence of intact bevasiranib in these tissues 24 h following intravitreal injection of non-radiolabeled bevasiranib (2 mg/eye). These studies demonstrate distribution of bevasiranib throughout the eye following intravitreal injection, including extensive uptake into the retina.