May 2009
·
7 Reads
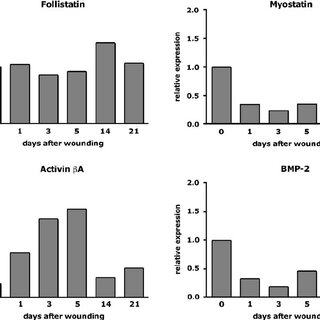
Wound healing in hpm mice. (A) Morphometrical analysis of wound healing parameters of 5 day wounds. Slightly reduced wound closure in hpm mice (control: n = 19, N = 7; hpm: n = 21, N = 7). The area of the hyperproliferative epithelium (HE) was similar in 5 day wounds of control and hpm mice (control: n = 14, N = 6; hpm: n = 11, N = 6). (B) Morphometrical analysis of wound healing parameters 13 days after wounding. The wound width was significantly increased (p = 0.0005; control: n = 16, N = 5; hpm: n = 16, N = 5) and the epidermis still hyperthickened in 13 day wounds of hpm mice (p = 0.0013; control: n = 19, N = 7; hpm: n = 21, N = 7). (C) 13 day wounds were examined for lacZ expression. Black arrowheads indicate the edges of the wound, white arrowheads mark an area of lacZ negative keratinocytes in the middle of the wound epithelium (control: N = 3; hpm: N = 4). Boxed area is shown at higher magnification. n, number of measurements; N, number of mice. (6.50 MB TIF)