Content uploaded by Graziella Messina
Author content
All content in this area was uploaded by Graziella Messina
Content may be subject to copyright.
ARTICLES
NATURE CELL BIOLOGY VOLUME 9 | NUMBER 3 | MARCH 2007 255
Pericytes of human skeletal muscle are myogenic
precursors distinct from satellite cells
Arianna Dellavalle
1,12
,
Maurilio Sampaolesi
1,2,12
, Rossana Tonlorenzi
1
, Enrico Tagliafico
3
, Benedetto Sacchetti
4
,
Laura Perani
1
, Anna Innocenzi
1
, Beatriz G. Galvez
1
, Graziella Messina
1,5
, Roberta Morosetti
6
, Sheng Li
7
,
Marzia Belicchi
8
, Giuseppe Peretti
1
, Jeffrey S. Chamberlain
7
, Woodring E. Wright
9
,
Yvan Torrente
8
,
Stefano Ferrari
3
, Paolo Bianco
4,10
and Giulio Cossu
1,4,11,13
Cells derived from blood vessels of human skeletal muscle can regenerate skeletal muscle, similarly to embryonic mesoangioblasts.
However, adult cells do not express endothelial markers, but instead express markers of pericytes, such as NG2 proteoglycan and
alkaline phosphatase (ALP), and can be prospectively isolated from freshly dissociated ALP
+
cells. Unlike canonical myogenic
precursors (satellite cells), pericyte-derived cells express myogenic markers only in differentiated myotubes, which they form
spontaneously with high efficiency. When transplanted into severe combined immune deficient–X-linked, mouse muscular dystrophy
(scid–mdx) mice, pericyte-derived cells colonize host muscle and generate numerous fibres expressing human dystrophin. Similar
cells isolated from Duchenne patients, and engineered to express human mini-dystrophin, also give rise to many dystrophin-positive
fibres in vivo. These data show that myogenic precursors, distinct from satellite cells, are associated with microvascular walls in
the human skeletal muscle, may represent a correlate of embryonic ‘mesoangioblasts’ present after birth and may be a promising
candidate for future cell-therapy protocols in patients.
Satellite cells embody the main myogenic activity in adult muscle
1–3
,
but lack the ability to cross the muscle endothelium when delivered
systemically, and because of this limited migration, they must be injected
intra-muscularly every 2 mm
3
of the patient’s muscles
4
. Furthermore, the
large majority of injected cells are lost within the first day
5
. Additional
problems include the reduced proliferation potency of satellite cells from
dystrophic patients and the recent observation that in vitro expansion
reduces in vivo differentiation potency
6
.
The demonstration that other cell types can differentiate into skeletal
muscle in vitro or in vivo
7
has created an alternative possibility for the
cell therapy of muscular dystrophy. In this context, the identification
of myogenic precursors in the wall of the embryonic dorsal aorta in
birds and rodents suggested that similar cells could be found in human
postnatal microvascular walls
8
.
From a strictly applicative point of view, the ideal cell population
should be: present in easily accessible postnatal tissues; expandable in
vitro to the large number of cells presumably required for systemic treat-
ment (1 × 10
9
or more); easily transducible with viral vectors; able to
reach skeletal muscle through a systemic route; and should be able to dif-
ferentiate into skeletal muscle cells in vivo with high efficiency. Here, we
isolate and characterize parietal cells from the microvasculature of human
skeletal-muscle cells, and show that they fulfill all these criteria.
RESULTS
Isolation and in vitro expansion of cells from muscle biopsies
Ten biopsies from non-dystrophic patients and six from Duchenne
muscular dystrophy (DMD) patients, ranging in age from 15–78 years
(non-DMD) and 3–8 years (DMD) were used in this study. Fragments
of interstitial tissue containing vessels were dissected and plated on
collagen-coated dishes. After the initial outgrowth of fibroblasts, small
round and refractile cells were observed (Fig. 1a) that adhered poorly to
the substratum, and these were collected by gentle pipetting. On aver-
age, 1 × 10
4
cells (arbitrarily counted as population doubling 2) were iso-
lated from a fragment of tissue weighing approximately 200 mg. When
grown in standard media that supports proliferation of satellite cells or
mesenchymal stem cells, these cells rapidly (within two passages) enter
1
Stem Cell Research Institute, San Raffaele Scientific Institute, 58 Via Olgettina, 20132 Milan, Italy.
2
Department of Experimental Medicine, University of Pavia, 6
Via Forlanini, 27100 Pavia, Italy.
3
Department of Biomedical Sciences, University of Modena and Reggio Emilia, 287 Via Campi, 41100 Modena, Italy.
4
Institute of
Cell Biology and Tissue Engineering, San Raffaele Biomedical Science Park, 100/2 Via Castel Romano, 00128 Rome, Italy.
5
Department of Cellular and Developmental
Biology, University of Rome La Sapienza, 5 Piazza Aldo Moro, 00161 Rome, Italy.
6
Department of Neurology, Catholic University, 8 Largo A. Gemelli, 00168 Rome,
Italy.
7
Department of Neurology, University of Washington, 1959 N.E. Pacific Street, Seattle, WA 98195-7720. USA.
8
Department of Neurological Science, Ospedale
Maggiore Policlinico, University of Milan, 35 Via Francesco Sforza, 20122 Milan, Italy.
9
UT Southwestern Medical Center, Dallas, 5323 Harry Hines Blvd., Dallas, TX
75390-9039, USA.
10
Department of Experimental Pathology, University of Rome La Sapienza, 324 Via Regina Elena, 00161 Rome, Italy.
11
Department of Biology,
University of Milan, 26 Via Celoria, 20130 Milan, Italy.
12
These authors contributed equally to this work.
13
Correspondence should be addressed to G.C. or P.B. (e-mail: cossu.giulio@hsr.it; p.bianco@flashnet.it)
Received 7 December 2006; accepted 30 January 2007; published online 11 February 2007; DOI: 10/1038/ncb1542
print ncb1542.indd 255print ncb1542.indd 255 14/2/07 16:21:5814/2/07 16:21:58
256 NATURE CELL BIOLOGY VOLUME 9 | NUMBER 3 | MARCH 2007
ARTICLES
senescence. However, a culture system was devised (see Methods) in which
most cells maintained a triangular, refractile morphology (Fig. 1b) and a
high proliferation rate for approximately 20 population doublings, with a
doubling time of approximately 36 h (Fig. 1d). The proliferation rate was
largely independent of donor age, although initially more cells outgrew
from explants of young DMD patients. Using in vitro expansion, approxi-
mately 2 × 10
9
cells were obtained from 1 × 10
4
culture-initiating cells. This
number may be suitable for intra-arterial delivery in young patients, based
on a per kg comparison with the mouse model used previously
9
. After ~20
population doublings, large flat cells were observed at increasing frequency
Population doublings
Days in vitro
20
15
10
5
10 20 30
LB
H1299 (2500 ng)
H1299 (250 ng)
H1299 (25 ng)
Passage VIII
Passage XII
Pas
sage XIX
19
7.7
6.2
4.3
3.5
2.7
1.9
1.5
a
c
e
b
d
f
kb
123
Figure 1 In vitro characterization of human adult interstitial cells. (a) Phase-
contrast morphology of the cellular outgrowth of a fragment of interstitial
tissue containing a small vessel cultured from a biopsy of normal adult
human muscle. Round and refractile cells are visible on top of a layer of
fibroblast-like cells. (b) Phase-contrast morphology of a polyclonal population
isolated from an explant culture after 5 passages in vitro. (c) Karyotype of
human interstitial cells after 15 passages, showing an euploid number of
chromosomes. (d) Proliferation curves of two different normal (open symbols)
and two dystrophic (closed symbols) cell populations. (e) Telomerase
activity of human adult interstitial cells at passage VIII, XII and XIX. Human
carcinoma cells, HI299, are also shown as a positive control. Amount of
protein extract is indicated. The arrow indicates the first ladder of polymerase
addition product; the black arrowhead shows non-specific amplification
products present in all samples; the white arrowhead shows the internal TRAP
assay standard, used as a semi-quantitative reference band. LB, negative
control. (f) Average telomere length from cells at passage VIII, XII and XIX
(lanes 1, 2 and 3, respectively) showing progressive shortening. The scale
bars represent 10 µm in a and b.
print ncb1542.indd 256print ncb1542.indd 256 14/2/07 16:22:0014/2/07 16:22:00
NATURE CELL BIOLOGY VOLUME 9 | NUMBER 3 | MARCH 2007 257
ARTICLES
and the whole population rapidly underwent senescence. At both early and
late passages, cells maintained a diploid karyotype (Fig. 1c). Early passage
(passage VIII) cells showed a significant telomerase activity (telomeric
repeats amplification protocol, TRAP, approximately 5–10% that found in
H1299 reference cancer cells (Fig. 1e). At later passages, telomerase activ-
ity was no longer detected, thus explaining the occurrence of proliferative
senescence. Consistently, telomere length progressively shortened and by
passage XIX had reached a size typical of presenescent cells (Fig. 1f). To
examine tumorigenicity, 1 × 10
7
human cells were injected subcutane-
ously into 10 nude and 10 SCID mice, which were then maintained up
to 12 months after the injection with no visible tumour detectable at
autopsy (data not shown). When similar cells were derived from biopsies
of Duchenne patients, they showed identical morphology and culture
behaviour (Fig. 1d and data not shown).
Phenotype of human adult muscle interstitial cells
Gene expression profiling, on Affymetrix chips, of two polyclonal
populations of cells from biopsies of normal individuals and two from
Duchenne patients, revealed that these cells express pericyte markers
(annexin V, alkaline phosphatase, desmin, smooth muscle actin, vimen-
tin and PDGF receptor β) at high levels
10
; however, they do not express
M-cadherin, N-CAM, cytokeratins or neurofilaments (with the excep-
tion of nestin), or endothelial markers (such as CD31, CD34 and KDR).
Immunocytochemistry, RT–PCR and western blot analysis on cultured
cells confirmed the results from microarray analysis (Fig. 2a–h). Clones
from one of these populations also expressed these markers in the same
percentage (approximately 20% of the population expressed smooth
muscle actin (SMA) or desmin, 50% expressed neural-glial-2 chondroi-
tin sulphate proteglycan (NG2) and more than 90% expressed PDGFRβ),
which did not vary at successive passages (data not shown). Of note,
myogenic markers (MyoD, Myf5 and Myogenin) expressed in cultures
of myogenic precursors were not expressed in these cells, as assessed by
array analysis or RT–PCR (Fig. 2g), with the possible exception of Pax3,
which was expressed by both populations at very low levels.
When the expression of surface antigens was determined, pericyte-like
cells were: uniformly negative for CD31, CD34, CD45, CD62L, CD71,
CD106, CD117 and CD133; weakly positive for CD49b, CD63, CD90,
CD105 and CD146; and strongly positive for CD13 and CD44 (Fig. 2i
and data not shown). All these results are in agreement with data from
microarray analysis (data not shown).
In culture, cells obtained from DMD patients were indistinguishable
from cells derived from normal muscle for all of the parameters described
above, and microarray analysis highlighted only a small number of
genes that are differentially expressed (Fig. 2j and see Supplementary
Information, Fig. S1). Some inflammatory genes seemed to be upregu-
lated in DMD cells, whereas few genes (such as Ephrin B2 and α tropo-
myosin) were expressed at higher levels in normal cells; however, the
significance of these observations is unclear. Notably, two normal poly-
clonal populations (Fig. 2j lanes 3, 4) and two clones from one of these
populations (lanes 5, 6) all expressed similar profiles, further demon-
strating the homogeneity of the cell population selected by the explant
culture method.
Prospective isolation of pericytes from skeletal muscle
When studying bone morphogenic protein-2 (BMP-2)-induced
osteogenic differentiation, all human cells selected by our culture
conditions expressed ALP, and also expressed ALP in the absence of
BMP-2 (Fig. 3a). Futhermore, the small round cells that outgrew from
the primary explant also expressed ALP (inset in Fig. 3a). In adult skel-
etal muscle, only vessels are known to be positive for ALP
11
(Fig. 3b).
Double staining for the endothelial marker CD31 (PECAM) and ALP
clearly showed ALP-positive (blue) cells adjacent to the endothelium
(brown) as typical pericytes (Fig. 3c). Pericytes and satellite cells were
localized in vivo in their specific anatomical locations. Fig. 3d shows a
triple-stained section of human normal skeletal muscle: satellite cells
(green, anti-M cadherin antibody) were located underneath the fibre
basal lamina (magenta, anti-laminin antibody), whereas pericytes (red,
anti-ALP antibody) were localized underneath the vessel basal lamina.
This localization was confirmed by confocal microscopy (Fig. 3e),
showing fluorescent images superimposed on a phase-contrast image
of normal adult skeletal muscle, where ALP
+
cells (magenta) were adja-
cent to CD31
+
cells (green), but were clearly separated from the M-Cad
+
(red) cells. These observations strongly suggest that the human cells
that we expanded in culture were derived from pericytes. To formally
demonstrate this hypothesis, biopsies of human normal skeletal muscle
(from individuals aged 25 and 46 years) were enzymatically digested to
a single-cell population that was separated by a fluorescence activated
cell sorter according to the expression of ALP and CD56 (recognizing
N-CAM, which is expressed in satellite cells, but not in pericytes). The
ALP
+
–CD56
–
fraction accounted for 2–4% of the total population in two
separate experiments, wheras the ALP
–
–CD56
+
fraction accounted for 9–
11%. In both experiments, the double-positive fraction (ALP
+
–CD56
+
)
represented less than 0.1% of the total population (Fig. 4a), suggesting
that cells coexpressing pericyte and satellite cell markers are rare in vivo.
After sorting, both ALP
+
–CD56
–
and ALP
–
–CD56
+
fractions were cloned
by limiting dilution, and the number and phenotype of growing clones
was evaluated after 5 days (data not shown) or 2 weeks (Fig. 4b–f). More
than 90% of newly developing clones from the ALP
+
–CD56
–
fraction
(at 5 days) expressed ALP, but did not express Myf5. In contrast, the
large majority (>80%) of clones from ALP
–
–CD56
+
fraction expressed
Myf5, but not ALP. A minority of clones did not express either Myf5
or ALP, and are likely to represent fibroblasts (data not shown). After
two weeks, the majority of clones derived from the ALP
+
–CD56
–
frac-
tion expressed ALP, but not Myf5 (Fig. 4b). These clones also expressed
SMA and PDGFRβ (data not shown), similarly to bona fide pericytes.
However, some clones seemed heterogeneous, with cells expressing
ALP or Myf5 in variable proportions (20–60% and 10–30% of the cells,
respectively), and rarely expressed both markers in the same cell. Other
clones were observed at this time that did not express either ALP or Myf5
(likely to be fibroblasts). On the contrary, the majority of clones from the
ALP
–
–CD56
+
fraction seemed to express Myf5 and not ALP, similarly
to myogenic precursors (Fig. 4c), whereas other clones from this frac-
tion did not express either marker (fibroblasts). Also in this fraction, a
significant number of clones expressed both Myf5 and ALP in different
cells and in some case in the same cell (Fig. 4d). A quantitative analysis
of this experiment is shown in Fig. 4g. The nature of the heterogeneous
clones has yet to be explained. In polyclonal cultures of satellite cells,
ALP-expressing cells have been observed
12
. However, polyclonal popula-
tions of ALP
+
pericyte-derived cells never expressed MyoD or Myf5 at
detectable level, unless myogenic differentiation was triggered. Clonal
conditions may induce myogenic differentiation in a fraction of the
clone, but this has yet to be tested. Nevertheless, the cloning experiments
print ncb1542.indd 257print ncb1542.indd 257 14/2/07 16:22:0214/2/07 16:22:02
258 NATURE CELL BIOLOGY VOLUME 9 | NUMBER 3 | MARCH 2007
ARTICLES
MyoD
Myf5
Myogenin
Pax7
Pax3
ALP
GAPDH
Fi
broblasts
Satellite cells
N
N
DMD
DMD
12345
NG2
GAPDH
PDGFRβ
123456
SMA Desmin
PDGFRβ
a
de f g
h
i
j
b
c
NG2
CD34 CD133
CD146
CD13
CD45
CD44
CD31
CD49b
Count Count Count Count
10
0
10
1
10
2
10
3
10
4
10
0
10
1
10
2
10
3
10
4
10
0
10
1
10
2
10
3
10
4
10
0
10
1
10
2
10
3
10
4
10
0
10
1
10
2
10
3
10
4
10
0
10
1
10
2
10
3
10
4
10
0
10
1
10
2
10
3
10
4
10
0
10
1
10
2
10
3
10
4
100
75
50
25
0
100
75
50
25
0
100
75
50
25
0
100
75
50
25
0
100
75
50
25
0
100
75
50
25
0
100
75
50
25
0
100
75
50
25
0
92.55%
0.10%
0.60%
82.51%
41.11%
97.32%
0.30% 0.20%
Pericyte
Figure 2 Phenotype of human adult pericyte-derived cells.
(a–f) Immunofluorescence microscopy analysis with anti-SMA (a) and
anti-desmin (b) antibodies, indicating expression in approximately 10%
of the population. In some cases, the cells coexpress these two markers
(arrows). An anti-PDGFRβ (c) stains the majority of the cells at the cell
surface, as detailed in d and e (which also shows costaining with anti-SMA)
and anti-NG2 (f). Nuclei are stained with DAPI. (g) RT–PCR analysis of
the expression of MyoD, Myf5, Myogenin, Pax7, Pax3 and ALP in human
fibroblasts, satellite cells, normal (N) and DMD pericytes. Control GAPDH
is also shown. (h) Western blot analysis of NG2 proteoglycan and PDGFRβ
in extracts from pericytes isolated from normal (lanes 1, 2) and DMD
(lanes 3, 4) muscle. Human normal muscle extract is also shown (lane 5)
as a negative control. GAPDH is shown for sample normalization. (i) FACS
analysis of human pericyte-derived cells using a panel of CD antibodies
(CD34, CD133, CD44, CD146, CD31, CD13, CD49b and CD45).
(j) Microarray analysis showing significant genes differentially expressed
between DMD (lanes 1, 2) and normal (lanes 3, 4) polyclonal populations
of pericyte-derived cells. Lanes 5 and 6 show the profile of differentially
expressed genes in two individual clones from the polyclonal population
shown in lane 3. The scale bars represent 20 m in a–f.
print ncb1542.indd 258print ncb1542.indd 258 14/2/07 16:22:0314/2/07 16:22:03
NATURE CELL BIOLOGY VOLUME 9 | NUMBER 3 | MARCH 2007 259
ARTICLES
indicate that human muscle pericytes can be prospectively isolated and
can give rise to clones that maintain expression of a pericyte phenotype.
As described below, pericyte-derived cells can differentiate into skeletal
muscle. Therefore, we selected 10 clones from the ALP
+
–CD56
–
fraction
and 10 from the ALP
–
–CD56
+
fraction for in vitro expansion. Part of each
clone was stained for ALP activity and Myf5 expression, and part was
induced to differentiate. Six out of 10 ALP
+
–CD56
–
clones and 10 out of
10 ALP
–
–CD56
+
clones differentiated into myotubes when exposed to
low-serum medium (examples of myogenic differentiation in one clone
derived from each fraction are shown in Fig. 4e, f).
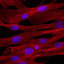
In vitro differentiation
ALP
+
–CD56
–
, pericyte-derived cells differentiate into smooth muscle,
osteoblasts or adipocytes after appropriate stimuli (data not shown).
When skeletal-muscle differentiation was induced by coculturing n-
LacZ labelled human adult pericyte-derived cells with mouse myogenic
cells, a very high percentage (more than 50%) of LacZ
+
nuclei fused
into hybrid myotubes (Fig. 5a, b) that expressed human MyoD (Fig. 5g).
Moreover, when exposed to muscle-differentiation medium, a large
proportion (ranging between 20 and 40% in different experiments) of
human adult pericyte-derived cells spontaneously differentiated into
myosin-positive multinucleated myotubes. No significant differences
were observed between cells from normal or DMD muscle (Fig. 5c,
d). Under similar conditions, approximately 60% of the myogenic cells
derived from normal satellite cells differentiated into multinucleated
myotubes (Fig. 5e). A quantitative analysis of these data is shown in
Fig. 5f. The morphological analysis was confirmed by western blot anal-
ysis, showing expression of sarcomeric myosin heavy chains in these
cultures (Fig. 5h). This result indicates a high skeletal myogenic poten-
tial for human pericyte derived cells. We then investigated the kinetics
of differentiation by measuring the expression of Pax7, Myf5, MyoD,
Myogenin and Myosin heavy chains in cells sorted for the expression
of CD56 or ALP (Fig. 6a). Pericyte derived cells (Fig. 6b
), at variance
with satellite cell-derived myogenic precursors (Fig. 6b), never expressed
Pax7, Myf5 or MyoD during the proliferation phase (Fig. 6d, Day 3 and
data not shown), but activated them at the onset of terminal differentia-
tion. The activation was simultaneous with myogenin (Fig. 6d, Day 5)
and shortly before the accumulation of myosin heavy chains in myo-
tubes (Fig. 6d, Day 7). Interestingly almost all MyoD
+
nuclei were inside
myosin heavy chain-positive myotubes. These data were quantified in
Fig. 6c. In contrast, satellite cell-derived myogenic precursors already
expressed Myf5 and Pax7 at the onset of the culture (Fig. 6f, Day 1)
Human cells Skeletal muscle
Skeletal muscle Skeletal muscle
b
a
ee′ e′′ e′′′
c
d
Figure 3 ALP activity in interstitial cells and muscle tissue. (a) Staining for
ALP indicates expression at varying levels in most of cells outgrown from
explants. The inset shows floating cells, just removed from the explant shown
in Fig. 1a, all of which also express ALP. (b) ALP activity in human normal
muscle, indicating expression in small vessels (arrow) of the interstitial
tissue. (c) Double staining of human normal muscle with an antibody against
PECAM, revealed by peroxidase staining in brown (arrowhead), and with
enzymatic reaction for ALP staining in purple (arrow). An endothelial cell
(arrowhead) is associated with a pericyte (arrow). (d) Immunofluorescence
microscopy of human normal muscle stained with antibodies against M-
cadherin recognizing satellite cells (green arrows), laminin (magenta) and
ALP (red) recognizing pericytes (red arrows). Nuclei are stained in blue with
DAPI. (e–e) Immunofluorescence microscopy of human normal muscle
stained with antibodies against CD31 (e, green), M-cadherin (e, red) and
ALP (e, magenta). A merged image is shown in e. Nuclei are stained
in blue with DAPI. All the images are superimposed on a phase-contrast
image showing human muscle fibres and interstitial tissue. The scale bars
represent 20 µm in all panels.
print ncb1542.indd 259print ncb1542.indd 259 14/2/07 16:22:0514/2/07 16:22:05
260 NATURE CELL BIOLOGY VOLUME 9 | NUMBER 3 | MARCH 2007
ARTICLES
and activated myogenin before myosin heavy chain (Fig. 6f, Day3).
Furthermore, when myosin heavy chain-positive myotubes had devel-
oped, many MyoD
+
–myosin heavy chain-negative cells were still present
in the culture (Fig. 6f, Day 5). These data were quantified in Fig. 6e.
In vivo studies
We then examined the myogenic potency of human pericyte-derived
cells in scid–mdx, immunodeficient mice. When injected into the femo-
ral artery of female scid–mdx dystrophic mice
13
, male human adult cells
or murine embryonic mesoangioblasts colonized downstream mus-
cle. After 24 h quantitative PCR for the Y chromosome revealed that
approximately 10% of injected cells (both murine and human) could
be detected in downstream muscles and less than 1% in contra-lateral
muscles, with the remaining cells being localized mainly in filter organs
(Fig. 7a). When human satellite cell-derived myogenic precursors were
similarly injected into the femoral artery of scid–mdx mice, no signal
(over background) could be detected in downstream muscles (data not
shown), confirming our previous results in mice
9
.
One week after injection, many human nuclei were identified (by the
anti-human lamin A/C antibody), mainly outside the basal lamina. A
fraction (approximately 15%) of injected cells was actively proliferating
(Ki67
+
) — as shown in Fig. 7b, where human nuclei are indicated in red
and proliferating human cells (arrows) in yellow in the merged image.
Many human cells expressed NG2 (Fig. 7c–e); however, several human
Pc ? Pc ? FbFb
100
80
60
40
20
ALP
+
– CD56
–
fraction ALP
–
– CD56
+
fraction
Sat Sat
Number of clones
CD56 PE
10.7%
0.05%
3.6%
ALP FITC
10
4
10
3
10
2
10
1
10
0
10
0
10
1
10
2
10
3
bca
d
g
ef
ALP
–
Myf5
+
ALP
+
Myf5
–
ALP
+
Myf5
+
ALP
–
Myf5
–
ALP
–
Myf5
+
ALP
+
Myf5
–
ALP
+
Myf5
+
ALP
–
Myf5
–
Figure 4 Clonal analysis of cells isolated from muscle. (a) Isolation of
ALP- and CD56-positive cells from freshly dissociated human muscle.
ALP
+
cells (3.6% of the total population) were separated from CD56
+
cells
(10.7%) by FACS. (b) An ALP
+
–Myf5
–
clone derived from the ALP
+
–CD56
–
fraction of total muscle stained for ALP (cytoplasmic staining) and with
an anti-Myf5 antibody, revealed by peroxidase. (c) An ALP
–
–Myf5
+
clone
derived from the ALP
–
–CD56
+
fraction of total muscle stained for ALP and
with an anti-Myf5 antibody (nuclear staining after peroxidase-conjugated
second antibody). (d) An ALP
+
– Myf5
+
clone from the ALP
+
–CD56
–
fraction
stained for ALP and with an anti-Myf5 antibody. A cell expressing both
markers is indicated by the arrow. (e, f) Skeletal muscle differentiation
of an ALP
+
–CD56
–
(e) and of an ALP
–
–CD56
+
clone (f), after two weeks
in culture, followed by an additional week in low-serum differentiation
medium. Cells were stained with MF20 anti-sarcomeric myosin antibody
(red) and DAPI. (g) Total number of clones from the ALP
+
–CD56
–
and ALP
–
–CD56
+
fractions, stained after 15 days for ALP and Myf5. Pc, pericyte-
derived cells; ?, cells with undefined phenotype; Sat, satellite cells; Fb,
fibroblasts. The scale bars represent 20 µm in b–d and 50 µm in e and f.
print ncb1542.indd 260print ncb1542.indd 260 14/2/07 16:22:0614/2/07 16:22:06
NATURE CELL BIOLOGY VOLUME 9 | NUMBER 3 | MARCH 2007 261
ARTICLES
nuclei (approximately 3–5% of total human nuclei) could be detected
underneath the basal lamina expressing M-Cadherin, a specific marker
of satellite cells (Fig. 7f–h).
To examine the residual clonogenicity of transplanted ALP
+
–CD56
–
cells, muscles from scid–mdx mice were digested one week after injection.
Four percent of freshly dissociated cells were of human origin and 90% of
these were ALP
+
, whereas no human cell was Myf5
+
. When dissociated
cells were cultured for three days and then stained for lamin A/C and
Myf5, several cells expressing either one or the other antigen could be
detected (see Supplementary Information, Fig. 2a–c), but none express-
ing both markers. This indicates that human satellite cells (identified by
coexpression of Myf5 and M-Cadherin; see Supplementary Information,
Fig. 2d) were not present in the transplanted population. These cells
were cloned by limiting dilution and 45 clones were obtained (clon-
ing efficiency, approximately 2%): two were Myf5
+
(see Supplementary
Information, Fig. 2e–g); 41 were ALP
+
(see Supplementary Inforamtion,
Fig. 2h, j); and two were negative for both markers (data not shown). A
polyclonal culture with ALP
+
human cells and ALP
–
mouse cells is shown
in the Supplementary Information, Fig. 2k. When expansion of rescued
human clones was attempted, three out of 20 clones were propagated for
approximately 10 population doublings, indicating that only a minority
of the transplanted population maintains a high proliferative capacity
in vivo (data not shown).
Dystrophin production by transplanted cells
We finally examined whether transplanted cells would fuse into multinu-
cleated myofibres that expressed human dystrophin, one month after the
last of three intra-arterial transplantations into scid–mdx mice. Specific
staining of anti-Dys1, Dys2 and human-specific Dys3 antibodies was
determined by staining a section of a transplanted tibialis anterior only
with secondary antibody, which did not produce any staining (Fig. 8a,
a
) using our conditions (see Methods). When non-transplanted tibialis
anterior of 2-month-old scid–mdx mice was stained with anti-Dys1 or
Dys2 antibodies, several clusters of revertant fibres (ranging in number
between 5 and 15), were observed (see Fig. 8b, b
). After three con-
secutive injections
9
of normal pericyte-derived cells, large areas of the
injected muscle were reconstituted with fibres expressing human dys-
trophin (Fig. 8c, c
and Table 1). The number of dystrophin positive fibres
in the tibialis anterior of transplanted animals (stained with anti-Dys3
antibody) ranged between 200 and 450 in five different transplanted
Satellite cells
Pericytes
60
f
gh
a
de
bc
45
30
15
Percentage differentiation
Normal
DMD
123456
12345 6
7
MyoD
MyHC
β-tubulinGAPDH
Normal satellite cells
Normal pericytes
DMD pericytes
Figure 5 In vitro myogenic differentiation of human pericyte derived cells.
(a, b) Fusion of pericyte-derived human cells, previously transduced with a
lentiviral vector expressing nuclear LacZ after coculture with mouse C2C12
myoblasts. Human LacZ
+
nuclei (arrowheads) are mainly detected inside
multinucleated, myosin positive myotubes. (c–f) Spontaneous differentiation
of normal (c) and dystrophic (d) human pericyte-derived cells, cultured in
differentiation medium on matrigel coated dishes. Normal human satellite
cell-derived myotubes are shown in e for comparison. A quantitative analysis
of the percentage of differentiation is shown in f. (g) RT–PCR with human
specific oligonucleotides for human MyoD expression in the coculture shown
in a and b. Lane 1, molecular markers; lane 2, C2C12 mouse myoblasts;
lane 3, human satellite cells; lanes 4–6, cocultures of C2C12 myoblasts
with different isolates of human pericyte-derived adult cells; lane 7, no
DNA. (h) Western blot analysis for the expression of sarcomeric myosin heavy
chains (MyHC) in normal (lanes 1, 3 and 5) and DMD (lanes 2, 4 and 6)
pericyte-derived human cells cultured in growth medium (lane 1, 2) and in
differentiation medium for one (lanes 3, 4) and eight (lanes 5, 6) days.
The scale bars represent 50 µm in a and b and 25 µm in c–e.
print ncb1542.indd 261print ncb1542.indd 261 14/2/07 16:22:0714/2/07 16:22:07
262 NATURE CELL BIOLOGY VOLUME 9 | NUMBER 3 | MARCH 2007
ARTICLES
mice. When human dystrophic cells, transduced in vitro with a lentivi-
ral vector expressing human mini-dystrophin, were similarly injected
into the skeletal muscle of scid–mdx mice, the results were similar to
those observed with normal cells (Fig. 8d, d
), with numbers of mini-
dystrophin-postive fibres ranging from 190 to 320 per crosssectional
area. In one experiment, three mice were transplanted intra-arterially
with a clonal population of ALP
+
pericyte-derived cells using the same
protocol as for polyclonal population. The number of dystrophin-posi-
tive fibres detected was similar to that observed with the polyclonal
population (Table 1). To compare the myogenic potential of pericyte-
derived cells with satellite cells, scid–mdx mice were transplanted with
three consecutive intra-arterial injections of human CD56
+
satellite cells.
e
cd
f
Myf5
GM 0
0
10
10
30
40
50
60
70
80
1234567
Pericyte-derived cells
Pax7
MyHC
Myogenin
MyoD
Days of differentiation
Percentage positve cellsPercentage positve cells
Satellite-derived myogenic cells
Myf5
Myogenin
MyHC
80
70
60
50
40
30
20
10
0
GM
01234567
Days of differentiation
Pax7
MyoD
Satellite-derived cells Pericyte-derived cells
CD56 PE
AP FITC
1.93%
0.07%8.78%
ab b′
Myog
MyHC
MyoD
MyHC
Myog
MyHC
MyoD
MyHC
Myf5
Pax7
Myf5
Pax7
Day 5
Day 3
Day 1
Day 7
Day 5
Day 3
Figure 6 Time course of myogenic differentiation in cultures of pericyte-derived
cells and satellite cell-derived myogenic precursors. (a) Cells were digested
from human skeletal muscle and FACS sorted into CD56
+
satellite cells
and ALP
+
pericytes, which were separately cultured in myogenic-promoting
conditions. (b, b) Phase-contrast morphology of the two cell types at day 1 in
culture are shown in b and b, respectively. (c–f) Positive cells were counted
in 20 randomly selected fields and calculated as percentage of total nuclei
visualized by DAPI. The time-course of expression of these different proteins
are shown for pericyte derived cells (c) and for satellite cells (e). Cultures were
fixed and stained daily with antibodies against Pax7, Myf5, MyoD, myogenin
and myosin heavy chains — examples are shown in d for pericyte-derived cells
and in f for satellite cells. The scale bars represent 20 µm.
print ncb1542.indd 262print ncb1542.indd 262 14/2/07 16:22:1014/2/07 16:22:10
NATURE CELL BIOLOGY VOLUME 9 | NUMBER 3 | MARCH 2007 263
ARTICLES
The number of dystrophin positive fibres detected in these animal was
very low (Table 1), and is likely to represent revertant fibres. These data
confirm that satellite cells cannot colonize muscle when systemically
delivered. For a more direct comparison, both pericyte-derived cells and
satellite cells were transplanted with a single intra-muscular injection
into the tibialis anterior scid–mdx mice. In this case, both populations
of cells gave rise to numerous dystrophin-positive fibres in the area of
injection, with satellite cells being more efficient than pericyte-derived
cells (Table 1). Finally, ALP
–
–CD56
–
fibroblasts did not give rise to sig-
nificant numbers of dystrophin-positive cells, independent from the
route of administration (Table 1). The amount of human dystrophin
expressed in muscles transplanted intra-arterially with pericyte-derived
Percentage total migrated cells
30
20
5
0
In vivo migration
scid–mdx
Quadriceps
Quadriceps
Gastrocnemius
Gastrocnemius
Tibialis
Tibialis
Liver
Spleen
Treated Contralateral
a b
c
f
e
d
gh
Figure 7 Tissue distribution of human pericyte-derived cells in dystrophic
muscle. (a) In vivo homing of 5 × 10
5
mouse male mesoangioblasts (blue
bars) or human (green bars) male pericyte-derived cells, injected into the
right femoral artery (treated muscles) of 2-month-old female scid–mdx mice.
After 24 h, different organs were collected and the percentage of migrated
cells was calculated by real-time PCR for the Y chromosome. A mean of three
independent experiments run in triplicate is shown. The error bars represent
s.d. (b) High magnification of human cells 7 days after transplantation in
the mouse muscle. Human lamin A/C-positive cells are visible in red and the
fraction of these cells that are proliferating are visible in yellow in the
merged image of the section (also stained with anti-Ki67 antibody). The
fluorescence image is superimposed on the phase-contrast image of the
tissue. (c–e) Triple-fluorescence images of transplanted human cells
coexpressing pericyte markers. Human nuclei stained with anti-lamin A/C and
DAPI appear violet (arrow), whereas mouse nuclei appear blue (c). Human
nuclei stained with anti-Lamin A/C (red) also express NG2 (magenta, d). NG2-
expressing pericytes (magenta) adjacent to CD31 expressing, endothelial
cells (green, e). (f–h) Human pericyte-derived cells localize underneath the
basal lamina and express the satellite cell marker M-Cadherin. Human nuclei
are stained with anti Lamin A/C antibody (arrows in f and h) and appear violet
in f (because of DAPI counterstain) and red in h. Satellite cells are stained
in green by an M-Cadherin antibody (g and h). The double arrow indicates
a human cell, expressing M-Cadherin, underneath the basal lamina that
is stained magenta by anti-laminin antibody (g), whereas the arrowhead
indicates a mouse satellite cell (g, h). Nuclei are counter-stained in blue with
DAPI (f, g). The scale bars represent 20 µm in all panels.
print ncb1542.indd 263print ncb1542.indd 263 14/2/07 16:22:1114/2/07 16:22:11
264 NATURE CELL BIOLOGY VOLUME 9 | NUMBER 3 | MARCH 2007
ARTICLES
cells was analysed by western blot (Fig. 8g), which revealed significant
accumulation of both normal and mini-dystrophin in the transplanted
quadriceps, although there were differences between different trans-
planted animals.
Functional analysis
SCID (n = 4), SCID–mdx-untreated mice (n = 3) and SCID–mdx mice
(n = 7), transplanted with human pericyte-derived cells (normal, n = 4;
DMD genetically corrected, n = 3), were tested for functional recovery
on a rotarod at a fixed speed of 1.6 m min
–1
up to 4 min. Results (see
Supplementary Information, Fig. S3a) showed that SCID mice fell in only
three times (after 320, 335 and 340 s) out of 12 tests, whereas untreated
SCID–mdx fell at all times, after running periods ranging from 30 to
160 s (nine out of nine). SCID–mdx mice, transplanted with normal
human pericyte-derived cells, fell 10 out of 12 times, and in two cases
completed the test (in the other cases they fell after periods ranging from
70–300 s). SCID–mdx mice, transplanted with DMD pericyte-derived
cells and transduced with the lenti-vector expressing human mini-dys-
trophin, fell seven out of nine times, after periods ranging between 60
and 310 s. Transplanted mice were also subjected to the exhaustion
treadmill, which measures muscle endurance — untreated SCID–mdx
mice showed a lower time of exhaustion in the this test compared with
SCID mice (see Supplementary Information, Fig. 3b). The groups receiv-
ing donor human pericyte-derived cells performed significantly better
than untreated dystrophic mice at all times, even though they did not
reach the level of activity of normal mice. These data showed a partial,
but significant, recovery of motility in mice transplanted with human
pericyte-derived cells.
DISCUSSION
This work describes the isolation of cells that can proliferate in vitro
from interstitial tissues of normal and dystrophic human skeletal muscle.
The cells can be expanded in vitro for about 20 population doublings
(up to numbers that would be sufficient to treat a paediatric patient),
transduced with viral vectors and induced to differentiate into skeletal
muscle. When transplanted into dystrophic immunodeficient mice, they
gave rise to large numbers of new fibres expressing human dystrophin,
thus fulfilling all the criteria required for a successful cell therapy for
muscular dystrophy.
Unexpectedly, we observed that cells outgrown from tissue explants
express ALP and a number of pericyte markers, and can be isolated from
pericytes — the only ALP
+
cells in skeletal muscle
11
. At variance with
embryonic mesoangioblasts, they do not express endothelial markers. A
possible interpretation of this difference may be found in the angioblastic
origin of mesoangioblasts
14
, which occupy an endothelial position in
the embryo and express some early endothelial markers. With further
development, these cells may move to a perithelial position, progressively
switching from an ‘endothelium-like’ to a ‘pericyte-like’ phenotype, simi-
lar to the phenotype observed in this work. Although it is possible that
the cells we isolated and characterized from pericytes may be the progeny
of prenatal mesoangioblasts, the lineage relationship may be complex
and cannot be determined in humans, where genetic labelling is not
feasible. Operationally, we define these cells as ‘pericyte-derived cells’.
Myogenic differentiation of pericyte-derived cells is strikingly high,
ranging from 20 to 40% in cells from different patients. This is approxi-
mately one order of magnitude more than that observed for other types
of stem cells, including mouse mesoangioblasts
8,9
. However, these cells
are clearly distinct from satellite cells as they have a number of unequivo-
cal characteristics: first, they have a different anatomical niche — peri-
cytes are located underneath the basal lamina of the small vessel, whereas
satellite cells are located inside the basal lamina of muscle fibres; sec-
ond, their growth requirements differ, as pericyte-derived cells undergo
rapid senescence in DMEM which is routinely to culture satellite cells;
third, satellite cells express MyoD, Pax7, Myf5, MEF 2C, CD56 and
M-cadherin, which are not expressed in pericyte-derived cells that
instead express NG2 and ALP (not expressed in satellite cells).
Interestingly, pericyte-derived cells express MyoD and Myf5 only on
terminal differentiation, suggesting distinct kinetics of myogenic dif-
ferentiation. Moreover, pericyte-derived cells can cross the vessel wall
— a feature presumed to be absent in satellite cell-derived myogenic
precursors. In 1992, it was reported that myogenic cells can be deliv-
ered arterially
15
, but this crucial experiment was never repeated despite
Table 1 Human cell types injected into irradiated scid–mdx host mice
Donor cell types
a
Delivery
b
Number of injected cells
Days after
injection
Number of animals
Number of dystrophin-
positive fibres per muscle
(mean ± s.e.m.)
c
None,
revertant fibres
NA – – 3 1.6 ± 0.6
Pericytes
(ALP
+
–CD56
–
)
i.a. (×3)
1.5 × 10
6
i.a.
(5 × 10
5
×3)
28 3 430.0 ± 45
Pericytes
(ALP
+
–CD56
–
; single clone)
i.a. (×3)
1.5 × 10
6
i.a.
(5 × 10
5
×3)
28 3 372.0 ± 43
Pericytes
(ALP
+
–CD56
–
)
i.m. 5–6 × 10
5
28 3 17.6 ± 5.0
Satellite cells
(ALP
–
–CD56
+
)
i.m. 5–6 × 10
5
28 3 42.6 ± 11.5
Satellite cells
(ALP
–
–CD56
+
)
i.a. (×3)
1.5 × 10
6
i.a.
(5 × 10
5
×3)
28 3 1.2 ± 0.8
Fibroblasts
(ALP
–
–CD56
–
)
i.m. 5–6 × 10
5
28 3 0.3 ± 0.2
Fibroblasts
(ALP
–
–CD56
–
)
i.a. (×3)
1.5 × 10
6
i.a.
(5 × 10
5
×3)
28 3 0.3 ± 0.1
a
Cells were injected at between 10 and 20 population doublings, whereas clones were injected at 25 population doublings.
b
NA, not available; i.m., single intra-muscular injection
into tibialis anterior; i.a., three consecutive intra-femoral artery injections.
c
In all injectedanimals, only the tibialis anterior was analysed.
print ncb1542.indd 264print ncb1542.indd 264 14/2/07 16:22:1314/2/07 16:22:13
NATURE CELL BIOLOGY VOLUME 9 | NUMBER 3 | MARCH 2007 265
ARTICLES
the obvious importance of systemic delivery. We found that arterially
delivered satellite cell-derived myogenic precursors were found inside
the vessels, but not outside of them
9
.
A detailed comparison of human adult pericyte-derived cells with
mesenchymal stem cells (MSCs)
16
, that will be reported elsewhere,
showed that the two cell populations clearly differ in the expression of
a significant number of genes. Moreover, human pericyte-derived cells
do not grow in α-MEM, the medium used for MSCs. Finally, MSCs
cannot differentiate into skeletal muscle spontaneously and do so at low
frequency only after treatment with 5-N-cytidine
17
.
NNmdxmdx
12345 678
g
aa′ bb′
cc′ dd′
ee′ ff′
Figure 8 Immunofluorescence microscopy and western blot analysis of
scid–mdx mouse tibialis anterior, after three serial transplantations of 5 × 10
5
human normal pericyte-derived cells and stained with antibodies against
laminin (green) and human dystrophin (Dys1/Dys2 or Dys3, red). (a, a)
Section stained with secondary antibody only. (b, b) Immunofluorescence
microscopy analysis of non-transplanted scid–mdx mouse tibialis anterior
stained with anti-Dys1 and Dys2 antibodies. A cluster of revertant fibres is
clearly evident (arrow). (c, c) Section of scid–mdx mouse tibialis anterior,
after three serial transplantation of 5 × 10
5
human normal pericyte-derived
cells. Many dystrophin-positive fibres are present throughout the section and
a dystrophin-negative area is indicated by the white line. (d, d) Section of
scid–mdx mouse tibialis anterior, after three serial transplantations of 5 × 10
5
human DMD pericyte-derived cells (in vitro transduced with a lentiviral vector
expressing human mini-dystrophin) after staining with antibodies against
laminin (green) and human dystrophin. Many mini-dystrophin positive fibres
are present throughout the section and a negative area is indicated by dashed
line. (e, e) Section of scid–mdx mouse tibialis anterior, after intra-muscular
injection of 5 × 10
5
human satellite cells. Many dystrophin-positive fibres
are present in the area of injection. (f, f) Section of scid–mdx mouse tibialis
anterior, after intra-muscular injection of 5 × 10
5
human fibroblasts. No
dystrophin-positive fibres were detected. (g) Western blot analysis of human
dystrophin expressed in muscles from different mice transplanted with
5 × 10
5
human normal pericyte-derived cells (lanes 1–5) and with DMD
human pericyte-derived cells, transduced with a lentiviral vector expressing
human mini-dystrophin, (lanes 6–8). Normal and DMD skeletal muscle are
shown as controls. The black arrow indicates wild-type dystrophin, the red
arrow indicates mini-dystrophin and the green arrow indicates myosin heavy
chains, shown as loading control. The scale bars represent 100 µm in a–f.
print ncb1542.indd 265print ncb1542.indd 265 14/2/07 16:22:1414/2/07 16:22:14
266 NATURE CELL BIOLOGY VOLUME 9 | NUMBER 3 | MARCH 2007
ARTICLES
In the past few years, many different types of mesodermal stem cells
have been isolated from both mouse and human tissues, and charac-
terized to different extents. These include: endothelial precursor cells
(EPCs)
18
; multipotent adult precursor cells (MAPCs)
19
; muscle derived
stem cells (MDCSs)
20
; side population cells
21–23
; Ac133
+
cells
24
; mesoan-
gioblasts
8
; and stem and/or precursor cells from muscle endothelium
25
,
sinovium
26
,
dermis
27
, and adipose tissue
28
. Different experimental proce-
dures, different sources and partial characterization still prevent a com-
plete understanding of the heterogeneity of these cells, and even less is
known of their origin and possible lineage relationships. Whatever the
case, many of these cells have been shown to differentiate into skeletal
muscle in vitro (MDSCs have also been shown to differentiate in vivo).
Some of these cells grow extensively in vitro, but others (such as EPCs
and side population cells) do not. Furthermore, EPCs and side popula-
tion cells can circulate, whereas systemic delivery has not been exam-
ined for most of the other cell types. Currently, human pericyte-derived
cells are the only cell type for which all the requested criteria have been
validated, although it is possible that other mesodermal stem cells may
show similar features —for example, cells isolated from adipose tissue
give rise to a few human dystrophin-expressing fibres when injected into
mdx mice
28
. Also, MSCs transduced with the intracellular active domain
of Notch and exposed to certain cytokines give rise to numerous fibres
in vitro and in the mdx muscle
29
. This result is intriguing, but awaits
a molecular explanation of the paradoxical effect of Notch, a known
inhibitor of myogenesis
30
that also has transforming ability
31
.
In future clinical protocols, systemic delivery seems to be an obligate
choice, as intra-muscular delivery would require an excessive number
of injections. Pericyte-derived human cells express some of the proteins
that leukocytes use to adhere to and cross the endothelium (that is, β2
and α4 integrins), and thus can diffuse into the interstitium of skeletal
muscle when delivered intra-arterially (a distinct advantage over resident
satellite cells that cannot).
Moreover, their extensive, but not indefinite, in vitro proliferation and
the maintenance of normal karyotype and myogenic potency, indicates
that human adult pericytes from a single biopsy may generate enough
cells to treat a paediatric patient with minimal risk of malignant trans-
formation. Importantly, dystrophic cells show the same proliferation
ability of their normal counterparts, suggesting that the disease has not
exhausted their growth potency, at least at a young age.
In conclusion, we have shown that pericytes represent a second myo-
genic precursor, resident in adult human skeletal muscle, with similar
myogenic potency to, but phenotypically distinct from, satellite cells.
Because of these features, pericyte-derived cells are an ideal cell popula-
tion for future cell therapy of muscular dystrophy.
METHODS
Isolation and culture of human adult pericyte-derived cells. Cells were pre-
pared from ten patients undergoing diagnostic biopsy and later classified as
non-dystrophic (and non affected by secondary myopathies) and from six DMD
patients, ranging in age from 15–78 years (non DMD) and 3–8 years (DMD).
The muscle samples (100–200 mg) from needle biopsies of the biceps brachialis
were stored in DMEM w/o FCS, with antibiotics and kept at 4 °C for maximum
24 h before dissection. The muscle samples were rinsed in PBS with Ca
2+
–Mg
2+
and sharply dissected into 1–2 mm diameter pieces with a scalpel. Fragments of
interstitial tissues containing small vessels were transferred to a Petri dish coated
with type I collagen (1 mg ml
–1
in 0.1 M acetic acid). The medium consisted of
MegaCell DMEM (Sigma, St Louis, MO) supplemented with 5% FCS, 5 ng ml
–1
basic fibroblast growth factor, 2 mM glutamine, 0.1 mM β-mercaptoethanol, 1%
non essential aminoacids, 100 IU ml
–1
penicillin and 100 mg ml
–1
streptomy-
cin. The tissue fragments were cultured for 7–8 days. After the initial outgrowth
of fibroblast-like cells, small round and refractile cells were observed (Fig. 1a).
Because of their poor adhesion (many of these cells were floating), this cell popu-
lation was easily collected by gently pipetting of the original culture and was plated
on collagen-coated dishes at a density of 5 × 10
4
cells per 30-mm dish. The cells
were either grown as a polyclonal population or cloned by limiting dilution on
collagen-coated dishes.
Satellite cell and fibroblast cultures. Satellite cells were isolated from biop-
sies of human skeletal muscle, as routinely performed in our laboratory
32
(see
Supplementary Information, Methods).
Cell cloning. Cells isolated from either explants or enzymatic digestion were
stained with Trypan blue, and living cells (excluding the dye) were counted in a
haemocytometer. The cell suspension was cloned by limiting dilution in the same
medium used for mass culture.
Flow cytometry and sorting. Cells were isolated from normal and DMD muscle
and analysed by flow cytometry or separated through a fluorescence activated
cell sorter (see Supplementary Information, Methods).
Analysis of cell proliferation. Cells were plated at a density of 5 × 10
3
cells per
cm
2
in different media, and passed on average every three days. At each passage,
the number of cells was counted in triplicate in a haemocytometer.
Karyotype analysis. All cell isolates from each individual patient were karyotyped
at an early and at a late passage. Cells, plated at one third confluence 72 h before
analysis, were processed with the Karyomax kit (Invitrogen, Paisley, UK) accord-
ing to the manufacturer’s instructions. For each of the karyotypes analysed, five
different metaphase spreads were examined.
Telomerase activity and telomere-length analysis. Telomerase activity was
determined in three different samples using the TRAP assay, as described previ-
ously
33
. Telomere length was measured after DNA extraction from cell samples
with different population doublings by digestion with the restriction enzymes
AluI, CfoI, HaeIII, HinfI, MspI and RsaI, and electrophoresis on 0.7% agarose gels
as previously described
34
. The gels were denatured, dried and neutralized, and the
signal was detected in situ using a telomeric probe end-labelled with
32
P-ATP.
Tumorigenicity. To test for possible tumour formation, 10 nude and 10 SCID
mice were injected subcutaneously each with 1 × 10
7
human pericyte-derived
cells from two non-DMD (age 15, 40) patients (five nude and five SCID each),
and maintained for one year after the injection. The same number of mice were
similarly each injected with 1 × 10
7
pericyte-derived cells from two DMD patients
(age 3 and 6), previously transduced with a lentiviral vector expressing human
mini-dystrophin. After 12 months, the mice were killed and analysed for the
presence of macroscopically detectable tumours.
Cell transduction with lentiviral vectors. Cells were transduced, as previously
described
9
, with third-generation lentiviral vectors expressing nuclear LacZ or
human mini-dystrophin
35
.
Differentiation assays. Differentiation into smooth muscle cells and osteoblasts
was induced by treatment with TGFβ-1 and BMP2, respectively, as previously
described
8
. Differentiation into skeletal muscle cells was induced by cocultur-
ing human adult pericyte-derived cells (previously transduced with a lentivector
expressing n-LacZ) with C2C12 mouse myoblasts at 1:5 ratio (see Supplementary
Information, Methods).
Spontaneous skeletal myogenic differentiation of human pericyte-derived
cells was induced by plating cells onto matrigel-coated dishes in differentiation
medium. After 7 days, cultures were fixed and stained with antibodies against stri-
ated myosin (MF20) and MyoD. Western blot analysis was performed using the
same antibodies. Human satellite cells, used as a positive control, were cultured
as previously described
32
.
Immunofluorescence microscopy. Cells were grown on matrigel-coated glass
coverslips for 2 days at an initial concentration of 2 × 10
4
per coverslip), washed
with PBS and fixed with 4% paraformaldehyde for 10 min. Muscle samples from
print ncb1542.indd 266print ncb1542.indd 266 14/2/07 16:22:1714/2/07 16:22:17
NATURE CELL BIOLOGY VOLUME 9 | NUMBER 3 | MARCH 2007 267
ARTICLES
control, or cell-transplanted scid–mdx mice were frozen in liquid nitrogen-cooled
isopentane and serial 8 µm-thick sections were cut with a Leyca cryostat. Cells and
tissue sections were processed for immunofluorescence microscopy as previously
described
8
(see Supplementary Information, Methods).
Immunoblotting and antibodies. Western blotting analysis of cells and tissues
was performed as previously described
8,9
. The antibodies used in this study are
described in the Supplementary Information, Methods.
In vivo transplantation. Approximately 5 × 10
5
human pericyte-derived cells
and mouse D16 mesoangioblasts (both male) were injected into two-month-old
female scid–mdx dystrophic mice, as previously described
9
. Animals were killed
at different times after the injection. To measure the fraction of injected cells
retained into skeletal muscles, animals were sacrificed 24 h after the injection,
and different muscles (quadriceps, gastrocnemius and tibialis anterior) or filter
organs (liver, lung and spleen) were collected. RNA was extracted and a real-time
PCR for the Y chromosome was performed in all the samples, as described else-
where. Data are represented as percentage of cells (percentage of Y chromosome
detected) migrated to the different organs relative to the input value. To analyse
human dystrophin expression, three consecutive injections at 30 day intervals
were performed, and animals were sacrificed 20 days after the last injections. The
injected and non-injected tibialis anterior were processed for immunofluores-
cence microscopy, whereas a membrane fraction was purified from the injected
quadriceps of the same mice, separated of 6% SDS–PAGE and analysed by western
blot, as previously described
8
. Human satellite cells and fibroblasts were injected
intra-arterially following the same protocol.
Alternatively, 5 × 10
5
pericyte-derived cells (ALP
+
–CD56
–
), satellite cells
(ALP
–
–CD56
+
) or fibroblasts (ALP
–
–CD56
–
) were suspended in 10 l PBS
and injected intra-muscularly in the tibialis anterior of scid–mdx mice, as
previously described
32
.
Gene-expression profiling and data analysis. Gene expression profiling analysis
was conducted on total cellular RNA isolated from different cell populations: two
DMD patients, age 3 and 6; two healthy individuals, age 15 and 40; and from two
clones of the 40 years old individual (see Supplementary Information, Methods).
Exercise protocols. Control, dystrophic, and dystrophic transplanted mice were
subjected to functional analysis using a rotarod and treadmill (see Supplementary
Information, Methods).
Note: Supplementary Information is available on the Nature Cell Biology website.
ACKNOWLEDGEMENTS
This work was supported by grants from Muscular Dystrophy Association (MDA),
Telethon, Association Française contra les Myopathies (AFM), Parent Project
Onlus, Cassadi Risparmio Province Lombarde (CARIPLO), Associazione Italiana
ricerca sul Cancro (AIRC), EC ‘Eurostemcell’, ‘Cellsintoorgan’, MyoAmp and
‘Genostem’, and the Italian Ministries of Health and Research. We thank G. Arrigo
for help with karyotype analysis and A. Palini for help with FACS analysis. We also
thank E. Dejana for advice and for reading the manuscript.
AUTHOR CONTRIBUTIONS
A.D. and M.S. coordinated the work and performed the in vivo transplantation
and functional tests. R.T. performed the cell cultures with help from G.M. and
R.M. E.T. and S.F. conducted the microarray analysis. B.S. and A.D. did the FACS
work. L.P. performed the PCR and western blot analysis. A.I. and M.B. did the
immunocytochemistry. B.G.G. performed the homing experiment. S.L. and J.S.C.
provided the viral vectors and advice. G.P. and Y.T. provided the biological samples.
W.E.W. performed the telomerase work, provide advice and revised the manuscript.
P.B. and G.C. coordinated the whole project and wrote the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare that they have no competing financial interests.
Published online at http://www.nature.com/naturecellbiology/
Reprints and permissions information is available online at http://npg.nature.com/
reprintsandpermissions/
1. Mauro, A. Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 9, 493–
495 (1961).
2. Sherwood, R. I. et al. Isolation of adult mouse myogenic precursors: functional hetero-
geneity of cells within and engrafting skeletal muscle. Cell 119, 543–554 (2004).
3. Collins, C. A. et al. Stem cell function, self-renewal, and behavioral heterogeneity of
cells from the adult muscle satellite cell niche. Cell 122, 289–301 (2005).
4. Morgan, J. E. et al. Long-term persistence and migration of myogenic cells injected
into pre-irradiated muscles of mdx mice. J. Neurol. Sci. 115, 191–200 (1993).
5. Beauchamp, J. R., Morgan, J. E., Pagel, C. N. & Partridge, T. A. Dynamics of myoblast
transplantation reveal a discrete minority of precursors with stem cell-like properties
as the myogenic source. J. Cell Biol. 144, 1113–1121 (1999).
6. Montarras, D. et al. Direct isolation of satellite cells for skeletal muscle regeneration.
Science 309, 2064–2067 (2005).
7. Cossu, G. & Sampaolesi, M. New therapies for muscular dystrophy: cautious optimism?
Trends Mol. Med. 10, 516–520 (2004).
8. Minasi, M. G. et al. The meso-angioblast: a multipotent, self-renewing cell that
originates from the dorsal aorta and differentiates into most mesodermal tissues.
Development 129, 2773–2783 (2002).
9. Sampaolesi, M. et al. Cell therapy of α sarcoglycan null dystrophic mice through intra-
arterial delivery of mesoangioblasts. Science 301, 487–492 (2003).
10. Armulik, A., Abramsson, A. & Betsholtz, C. Endothelial/pericyte interaction. Circ. Res.
97, 512–523 (2005).
11. Safadi, A., Livne, E., Silbermann, M. & Reznick, A. Z. Activity of alkaline phosphatase
in rat skeletal muscle localized along the sarcolemma and endothelial cell membranes.
J. Histochem. Cytochem. 39, 199–203 (1991).
12. Katagiri, T. et al. Bone morphogenetic protein-2 converts the differentiation path-
way of C2C12 myoblasts into the osteoblast lineage. J. Cell Biol. 127, 1756–1766
(1994).
13. Galvez, B. G. et al. Complete repair of dystrophic skeletal muscle by mesoangioblasts
with enhanced homing ability. J. Cell Biol. 174, 231–243 (2006).
14. Cossu, G. & Bianco, P. Mesoangioblasts, vascular precursors for extra-vascular meso-
derm. Curr. Opin. Genet. Develop. 13, 537–542 (2003).
15. Neumeyer, A. M. et al.
Arterial delivery of myoblasts to skeletal muscle Neurology 42,
2258–2262 (1992).
16. Bianco, P. & Gehron Robey, P. Marrow stromal stem cells. J. Clin. Invest. 105, 1663–
1668 (2000).
17. Wakitani, S., Saito, T. & Caplan, A. I.
Myogenic cells derived from rat bone marrow
mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve 12, 1417–1426
(1995).
18. Asahara, T. et al. Isolation of putative precursor endothelial cells for angiogenesis.
Science 275, 964–967(1997).
19. Reyes, M. et al. Purification and ex vivo expansion of postnatal human marrow meso-
dermal precursor cells. Blood 98, 2615–2625 (2001).
20. Qu-Petersen, Z. et al. Identification of a novel population of muscle stem cells in mice:
potential for muscle regeneration J. Cell Biol. 157, 851–864 (2002).
21. Asakura, A., Seale, P., Girgis-Gabardo, A. & Rudnicki, M. A. Myogenic specification of
side population cells in skeletal muscle. J. Cell Biol. 159, 123–134 (2002).
22. LaBarge, M. A. & Blau, H. M. Biological progression from adult bone marrow to mono-
nucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell 111,
589–601 (2002).
23. Bachrach, E. et al. Systemic delivery of human mini-dystrophin to regenerating mouse
dystrophic muscle by muscle precursor cells. Proc. Natl Acad. Sci. USA 101, 3581–
3586 (2004).
24. Torrente, Y., et al. Human circulating AC133
+
stem cells replenish the satellite cell
pool, restore dystrophin expression and ameliorate function upon transplantation in
murine dystrophic skeletal muscle. J. Clin. Invest. 114, 182–195 (2004)
.
25. Tamaki. T. et al. Identification of myogenic-endothelial precursor cells in the interstitial
spaces of skeletal muscle. J Cell Biol. 157, 571–577 (2002).
26. De Bari, C. et al. Skeletal muscle repair by adult human mesenchymal stem cells from
synovial membrane. J Cell Biol. 160, 909–918 (2003).
27. Toma, J. G. et al. Isolation of multipotent adult stem cells from the dermis of mam-
malian skin. Nature Cell. Biol. 3, 778–784 (2001).
28. Rodriguez, A. M. et al. Transplantation of a multipotent cell population from human
adipose tissue induces dystrophin expression in the immunocompetent mdx mouse. J.
Exp. Med. 201, 1397–1405 (2005).
29. Dezawa, M. et al. Bone marrow stromal cells generate muscle cells and repair muscle
degeneration. Science 309, 314–317 (2005).
30. Luo, D., Renault, V. M. & Rando, T. A. The regulation of Notch signaling in muscle stem cell
activation and postnatal myogenesis. Semin. Cell. Dev. Biol. 16, 612–622 (2005).
31. Leong, K. G. & Karsan, A. Recent insights into the role of Notch signaling in tumorigenesis.
Blood 107, 2223–2233 (2005).
32. Lattanzi, L. et al. High efficiency myogenic conversion of human fibroblasts by adeno-
viral vector-mediated MyoD gene transfer. J. Clin. Invest. 101, 2119–2128 (1998).
33. Wright, W. E., Shay, J. W. & Piatyszek, M. A. Modifications of a telomeric repeat ampli-
fication protocol (TRAP) result in increased reliability, linearity and sensitivity. Nucleic
Acids Res. 23, 3794–4005 (1995).
34. Ouellette, M. M. et al. Subsenescent telomere lengths in fibroblasts immortalized by
limiting amounts of telomerase. J. Biol. Chem. 275, 10072–10076 (2000).
35. Li, S. et al. Stable transduction of myogenic cells with lentiviral vectors expressing a
minidystrophin. Gene. Ther. 12, 1099–1108 (2005).
36. Irizarry, R. A. et al. Exploration, normalization, and summaries of high density oligo-
nucleotide array probe level data. Biostatistics 4, 249–264 (2003).
37. Liu, W. M. et al. Analysis of high density expression microarrays with signed-rank call
algorithms. Bioinformatics 18, 1593–1599 (2002).
38. Tusher, V. G., Tibshirani, R. & Chu,G. Significance analysis of microarrays applied
to the ionizing radiation response. Proc. Natl Acad. Sci. USA 98, 5116–5121
(2001).
print ncb1542.indd 267print ncb1542.indd 267 14/2/07 16:22:1814/2/07 16:22:18
SUPPLEMENTARY INFORMATION
WWW.NATURE.COM/NATURECELLBIOLOGY 1
Figure S1 Expression profiles of genes differentially expressed in two
populations of Duchenne (DMD3 and DMDA) and of normal (MIX40Y
and MIX78Y) human pericyte derived cells and in two clones isolate from
MIX78Y (CL9 and CLB) after IX PD in culture. Only those genes whose
expression vary at least 3 fold among all the Duchenne and normal cells are
shown. Values refer to the GCOS signal; black cells show transcripts with
an “absent” call; signals for transcripts with a “present” call are showed in
gradient coloured cells from blue (low abundant transcripts) to red (very high
abundant transcripts).
© 2007 Nature Publishing Group
SUPPLEMENTARY INFORMATION
2 WWW.NATURE.COM/NATURECELLBIOLOGY
Figure S2 Isolation and cloning of human pericyte derived cells previously
transplanted in dystrophic mouse muscle. (a-c) Three day culture of cells
isolated from transplanted muscle. Human cells are labeled by anti-Lamin
A/C in red, while satellite derived cells are labeled by anti-Myf5 antibody
in green and by anti-M Cadherin in red (d). No double labeled cells were
detected. (e,g) One clone of human (Lamin A/C+, red) cells that uniformly
expresses Myf5 (green) indicating derivation of a myogenic precursor from
transplanted human cells. (h,j) Two clones of human cells (Lamin A/C+,
brown after peroxidase staining) which express low (h) of high (j) level of
ALP (cytoplasmic staining). A mixed population of human ALP+ (arrow) and
mouse ALP- cells (arrowhead) is shown in k for comparison. Bar = 20 µm.
High magnification of cells indicated by arrows is shown in the inset.
© 2007 Nature Publishing Group
SUPPLEMENTARY INFORMATION
WWW.NATURE.COM/NATURECELLBIOLOGY 3
Figure S3 Exercise performance assessed by the rotarod test (a) and a run-
to-exhaustion protocol on a motorized treadmill (b). (a), SCID, SCID/mdx
untreated mice and SCID/mdx mice, transplanted with human perycyte-
derived cells (either normal or DMD but genetically corrected with mini-
dystrophin gene: mini-dys) were tested for functional recovery on a rotarod
at a fixed speed of 1.6 m/min up to 6 minutes. SCID mice felt only 3
times (after 320, 335 and 340 s) out of 12 tests (3/12) whereas untreated
SCID/mdx felt all the times, after running periods ranging from 30 to 160
s (9/9). SCID/mdx mice, transplanted with normal human perycyte-derived
cells, felt 10 out of 12 times (10/12) and in 2 cases completed the test;
in the other cases mice felt after periods ranging from 70 to 300 s. SCID/
mdx mice, transplanted with DMD perycyte- derived cells, transduced with
the lenti-vector expressing human mini-dystrophin, felt 7 out of 9 tests
(7/9), after periods ranging between 60 and 310 s. (b), SCID, SCID/mdx
untreated mice and SCID/mdx mice transplanted with human perycyte-
derived cells were subjected to a run-to-exhaustion protocol on a motorized
treadmill as described in Materials and Methods; n = 3 for each group; * p <
0.001 compared to corresponding SCID values; ** p < 0.005 compared to
corresponding SCID/mdx untreated (SCID/mdx) values.
© 2007 Nature Publishing Group
1
Supplementary Methods for NCB-CO9476E
Satellite cell and fibroblast cultures: Briefly, muscle fragments were digested with 2%
(w/v) collagenase II (Gibco BRL) for 60 min at 37°C. Digested cells were discarded and fragments
were incubated again with 0.05% trypsin (Gibco BRL) for 15 min at 37°C with gentle agitation. After
the incubation, isolated cells were collected and fragments were incubated again until the whole tissue
was digested (usually three times). The isolated cells were pooled, centrifuged and resuspended in
DMEM supplements with 20% pre-screened FCS, 1% gentamycin, and plated onto collagen coated
dishes at a density of 10
4
cells x cm
2
. Contamination by non myogenic cell was reduced by pre-plating
the cell suspension onto plastic dishes where fibroblasts tend to adhere more rapidly. Differentiation
was induced shifting the medium to DMEM supplemented with 2% horse serum. Fibroblasts were
prepared by subculturing cells outgrown from an explant of a dermis biopsy and grown in DMEM
supplemented with 20% FCS.
Flow cytometry and sorting: Three normal and three DMD biopsies were finely minced
and digested with collagenase/dispase (Sigma) digestion (0,8 IU/mg for 30 min at 37 C°). The cell
suspension was filter to remove tissue debris. Isolated cells were harvested and resuspended in 1
ml of a solution containing 5 µg/ml propidium iodide (Sigma) in PBS (Gibco Brl) and incubated
with specific antibodies for 1 hour at 4 ˚C; after PBS washing cells were fixed in 2% PFA before
FACS analysis. Cell analysis was performed on at least 10.000 events for each sample and
determined using a FACScalibur flow cytometer (Becton Dickinson) equipped with an argon laser
emission of 488 nm. FITC was identified by using a 530 band pass filter. The analysis was
performed using CELLQUEST software (Becton Dickinson). A primary gate based on physical
parameters (forward and side light scatter, FSC and SSC, respectively) was set to exclude dead cells
or small debris. The background level was estimated by omitting the primary antibody.
Before sorting, cells were incubated with sheep anti-human Alkaline phosphatase (Biogenesis) and
PE-conjugated mouse anti-human CD56 (Miltenyi Biotec) antibodies according to the
manufacture’s recommendations.
© 2007 Nature Publishing Group
2
Differentiation assays: Co-cultures were shifted to differentiation medium (DMEM
supplemented with 2% horse serum). After 7 days cultures were stained with antibodies against
striated myosin (MF20) and X-Gal. Percentage of myogenic differentiation was calculated by
counting the number of LacZ+ nuclei within myosin positive cells as percentage of total LacZ+
nuclei. Biochemical differentiation was confirmed by RT-PCR using human specific
oligonucleotides for MyoD (FW: CGATATACCAGGTGCTCTGAGGG; REV:
GGGTGGGTTACGGTTACACCTGC).
Immunofluorescence: Sections were incubated with primary antibodies overnight at 4°C in
PBS supplemented with 1% BSA and 0.2% TritonX-100. For the anti-Myf5 and M-Cadherin
antibodies (see below) sections were fixed in acetone for 10 min at -20°C and antibody incubation
was for 1 h at RT. For the M-Cadherin antibody, all solutions also contained 10 mM Ca
2+
. All
sections were washed x 3 in PBS and incubated with 10% donkey serum for 30 min at RT before
the addition of the appropriate Alexa 488 or Alexa 594 or Alexa 647 conjugated donkey secondary
antibodies. Background staining of anti-mouse secondary antibodies was reduced by addition of
0.1% mouse serum to the secondary antibody mixture. In some experiments we used biotinylated
secondary antibody from DAKO and processed according to the standard procedures of
VECTASTAIN Elite ABC kit (Vector Laboratories). After three final washes, the cover slips were
mounted on glass slides using mowiol in PBS and analyzed under a fluorescent microscope.
Antibodies: The following antibodies were used in this study: anti-dystrophin monoclonal
antibody Dys1, Dys2 and Dys3 (Novocastra) at 1:125 dilution; anti-laminin polyclonal rabbit
antibodies (Sigma) at 1:100 dilution; MF20 monoclonal antibody at 1:5 dilution, anti-smooth alpha
actin monoclonal at 1:300 dilution and anti-desmin rabbit polyclonal at 1:50 from Sigma, anti-Pax7
from the hybridoma bank at 1:3 dilution, anti-human lamin A/C 1:300 monoclonal from
Novocastra, anti-NG2 rabbit polyclonal at 1:250 (Chemicon), anti-PDGF receptor beta rabbit
polyclonal at 1:500 (from Cell Signaling Technologies); anti-M Cadherin goat polyclonal at 1:30
dilution and anti-Myf5 rabbit polyclonal at 1:200 dilution were from Santa Cruz; anti-PECAM rat
© 2007 Nature Publishing Group
3
monoclonal (a gift from Elisabetta Dejana) at 1:2 dilution. For FACS analysis the following
antibodies were used CD44, CD34, CD45, CD49b, CD117, CD62L,CD63,CD90 from BD
Biosciences, CD31, CD13, CD106 from ID labs inc, CD146 from Biocytes, CD105 from R&D
System and CD56 from Miltenyi Biotec. Secondary antibodies were from Molecular Probes.
Gene Expression Profiling and data analysis: Total cellular RNA was isolated from cell
populations (two DMD patients, age 3 and 6; two healthy individuals, age 15 and 40 and from two
clones of the 40 years old individual) using RNeasy RNA isolation kit (Qiagen, Valencia, CA)
following manufacturer’s recommendations. Disposable RNA chips (Agilent RNA 6000 Nano
LabChip kit) were used to determine the concentration and purity/integrity of RNA samples using
Agilent 2100 bioanalyzer. cDNA synthesis, biotin-labeled target synthesis, HG-U133 plus 2.0
GeneChip (Affymetrix, Santa Clara, CA) arrays hybridization, staining and scanning were
performed according to the standard protocol supplied by Affymetrix. The amount of a transcript
mRNA (signal) was determined by the Affymetrix GeneChip Operative Software (GCOS) 1.2
absolute analysis algorithm as already described
36
. All expression values for the genes in the GCOS
absolute analyses were determined using the global scaling option. Alternatively, probe level data
were converted to expression values using robust multi-array average (RMA) procedure
37
. Perfect
Match (PM) values were background adjusted, normalized using invariant set normalization, and
log transformed. The RMA generated data were uploaded onto GeneSpring
TM
software version 7.2
using the log2 transformation procedure. A “per chip” and a “per gene” normalization were
achieved by dividing each signal for the 50.0th percentile of all above -10 signals in that sample and
by the median of its values in all samples. A low-level filter in GeneSpring™ filtered out all those
probe sets called “Present” in less than 10% of samples or whose normalized expression levels were
always between 0.5 and 2 across all of the samples. For supervised analyses an initial filtering
procedure was applied in order to select transcripts showing a change call “I” or “D” in at least the
90% of the pair wise comparisons performed using the GCOS comparison algorithm
38
. Then,
supervised analyses were performed using an ANOVA test (Welch t-test at a confidence level of
© 2007 Nature Publishing Group
4
0.005) with the Benjamini & Hochberg correction of the family-wise error rate (FWER).
Hierarchical agglomerative clustering was performed in GeneSpring™ using Pearson’s correlation
coefficient and average-linkage as distance and linkage methods.
Exercise protocols: Control, SCID (n = 4), SCID/mdx untreated mice (n = 3) and
SCID/mdx mice, transplanted with human pericyte derived cells (normal, n = 4 and DMD,
genetically corrected, n = 3) were tested for functional recovery on a rotarod at a fixed speed of 1.6
m/min up to 4 minutes. The number of falls during this period was recorded. For the treadmill test,
mice (3 treated and 3 untreated SCID/mdx and 3 non dystrophic SCID) were adapted to the
procedure (10 min/day; 0.3 km/h) for 1 wk before beginning the exercise training protocol. Treated
and untreated mice were tested at a treadmill speed of 0.3 km/h. Treated animals were trained
during the transplantation period and tested 21 days after the last transplantation. Control animals of
the same age were tested at the same time.
© 2007 Nature Publishing Group