Content uploaded by Anna Trias Blasi
Author content
All content in this area was uploaded by Anna Trias Blasi on Feb 11, 2014
Content may be subject to copyright.
Accepted by Zhi-qiang Zhang: 28 Jan. 2014; published: 11 Feb. 2014 95
PHYTOTAXA
ISSN 1179-3155 (print edition)
ISSN 1179-3163 (online edition)
Copyright © 2014 Magnolia Press
Phytotaxa 159 (2): 095–104
www.mapress.com/phytotaxa/Article
http://dx.doi.org/10.11646/phytotaxa.159.2.3
A taxonomic revision of Pterisanthes (Vitaceae) in Thailand and a new Thai
record for Pterisanthes cissioides
Anna TRIAS-BLASI1,2,3*, Kongkanda CHAYAMARIT4, Atchara TEERAWATANANON5 & John A.N.
PA R NE L L 1,2
1Herbarium, Department of Botany, School of Natural Sciences, Trinity College Dublin, University of Dublin, D2, Ireland.
2Trinity Centre for Biodiversity Research, School of Natural Sciences, Trinity College Dublin, D2, Ireland.
3 Herbarium, Library, Art and Archives, Royal Botanic Gardens, Kew, Richmond, Surrey, TW9 3AB, U. K. (*Correspondence author:
a.triasblasi@kew.org).
4Forest Herbarium, Department of National Parks, Wildlife and Plant Conservation, Chatuchak, Bangkok 10900, Thailand.
5Thailand Natural History Museum, National Science Museum, Technopolis, Pathum
Thani 12120, Thailand.
Abstract
A revision of Pterisanthes in Thailand is presented with three species: P. eriopoda, P. polita and P. cissioides. Pterisanthes
cissioides Blume is newly recorded for Thailand. A description and illustration are provided. Full typification is presented
for all species and synonyms and lectotypes are selected for five names. Distribution maps are presented.
Introduction
The genus Pterisanthes Blume (Vitaceae) comprises ca. 19–20 species (Latiff 1982, 2001; Wen 2007) with a
distribution centred in Malaysia, extending northwards to Peninsular Thailand, Myanmar and the Philippines. In
many cases the distribution of Pterisanthes species is quite restricted, with five species restricted to the Malay
Peninsula, four to Borneo and two to Sumatra. In addition, around 5 species are only known from their type
location and one species (Pterisanthes pulchra Ridl.) is only found in the forest of Fraser’s Hill in Peninsular
Malaysia (Latiff 1987). Three Pterisanthes species are widespread in their distribution and have wide intraspecific
variation (Latiff 1982): Pterisanthes polita (Miq.) M.A. Lawson, Pterisanthes eriopoda (Miq.) Planch. and
Pterisanthes cissioides Blume.
The genus Pterisanthes was first described by Blume in 1825 from plants collected in Java that presented a
very distinctive inflorescence type. The genus is characterised by an inflorescence in the form of a leaf-opposed,
somewhat fleshy, leaf-like, flat lamellate panicle. A sterile tendril is present at the base of the peduncle (Trias-Blasi
et al. 2012). Often the lamellate flowers are partially immersed in the lamina. In 1858 Agardh suggested that
Pterisanthes should be placed in its own family, the Pterisanthaceae; however this suggestion was never adopted
(Latiff 1982). In 1863 Miquel transferred many Vitaceae species and genera to Vitis and then separated Vitis into
several sections. One of the genera transferred was Pterisanthes, becoming Vitis Sect. Pterisanthes, which at the
time contained five species. Even though authors such as Kuntze (1891) adopted Miquel’s classification system,
most authors agreed with Blume and retained generic status for Pterisanthes. Subsequently, new Pterisanthes
species were described by several authors: Lawson (1875) 3 spp.; Planchon (1887) 8 spp.; Ridley (1893, 1912,
1931) 4 spp.; Merrill (1907, 1917, 1929, 1934) 5 spp.; van Steenis & Bakhuizen van den Brink (1967) 2 spp.; Latiff
(1982) 3 spp. In 1982 Latiff separated the genus into two sections based on the nature of the inflorescences; while
Sect. Pterisanthes has pedicellate flowers on the margin of the lamellae, Sect. Paginiflora has sessile flowers.
Van Steenis & Bakhuizen van den Brink suggested (1967) Pterisanthes might have its origins in Ampelocissus
because of their shared morphological similarities such as seed morphology, petiole anatomy, indumentum types
and association of the tendril to the inflorescence (Latiff 1982). Recent molecular studies (Soejima & Wen 2006;
Wen et al. 2007; Trias-Blasi et al. 2012) have suggested relationships are with Ampelocissus, Nothocissus and Vitis.
TRIAS-BLASI ET AL.96 • Phytotaxa 159 (2) © 2014 Magnolia Press
In Thailand, the three species of Pterisanthes are restricted to the Peninsular floristic region (Figs. 1, 3, 4) and
are described here. During the preparation of the revision of the Vitaceae for the Flora of Thailand two specimens
from Phangnga and Narathiwat were discovered and were later identified as Pterisanthes cissioides Blume. This
species has been recorded in Peninsular Malaysia, Java, Borneo and Sumatra (Latiff 1982), but never in Thailand
until now.
Taxonomic treatment
Pterisanthes Blume (1825: 192). Type:—Pterisanthes cissioides Blume.
Embamma Griffith (1854: 694).
Vitis L. sect. Pterisanthes Miquel (1863: 73).
Slender wiry climbers. Stem slender, cylindrical, striate, glabrous. Leaves simple or palmately (2)3–7-foliolate,
some species heterophyllous. Inflorescence a leaf-opposed flat lamellate panicle, leaf-like and slightly fleshy with
associated tendrils at the peduncle; lamella shape very variable: horseshoe-shaped, rectangular, rounded to very
narrow. Flowers in Sect. Pterisanthes lamellate and pedicellate, in Sect. Paginiflora lamellate; lamellate flowers
hermaphrodite, sessile, partially embedded in the lamella, (3)-4-5-merous, calyx obscure, corolla petals ovate-
triangular, ovary adnate to the disk; pedicellate flowers placed at the margin of the lamella, either male or sterile
always 4-merous, calyx cupuliform; corolla petals ovate-oblong; filaments filiform, attached to the inner side of the
petal by a thin membrane; anthers minute; ovary exserted; style short; stigma inconspicuous. Seeds obovate,
rugose.
Distribution:—Brunei Darussalam, Indonesia (Java, Kalimantan, Sumatra), Malaysia (Peninsula, Sabah,
Sarawak), Myanmar (Peninsula), Philippines (Mindanao), Singapore, Thailand (Ranong, Songkhla, Narathiwat).
Key to Thai species
1. Pedicellate flowers absent; leaves with a ferruginous margin; petioles 1–3 cm long........................... Pterisanthes eriopoda
- Pedicellate flowers present; leaves without a ferruginous margin; petioles 3–7 cm long ..................................................... 2
2. Leaves simple and glabrous; lamella 1–3 cm wide .................................................................................... Pterisanthes polita
- Leaves compound with abaxial side ferruginous; lamella 3–7 cm wide .............................................Pterisanthes cissioides
Pterisanthes eriopoda (Miq.) Planchon (1887: 418). Vitis eriopoda Miquel (1863: 95). Pterisanthes ovata Korth.
nom. nud. Lectotype (designated here):—INDONESIA. Sumatra, Korthals s.n. (lectotype L! (electronic image
with barcode L0715762); isolectotype L! (electronic image with barcode L0715763) and K!).
P. coriacea var. araneosa King (1896: 408). Lectotype (designated here):—MALAYSIA. Peninsular Malaysia, Perak (central),
Gopeng Kinta, July 1883, King’s Collector 4621 (K!).
P. beccarina Planchon (1887: 418). Lectotype (designated here):—Sarawak, Beccari 796 (FI! (No: 2743); isolectotypes FI!
(No: 2743A), FI! (No: 2743B)).
Tendrils branching from the inflorescence peduncle, mostly simple, sometimes bifurcate, 5–15 cm long, slender,
slightly hairy or tendrils unrelated to the inflorescence, simple, robust, leaf-opposed. Leaves simple; petiole 1–3 cm
long, with long and thin (ca. 3 mm × 0.1 mm) wavy ferruginous hairs; leaf blade ovate to elliptical, to 19 × 10 cm,
base cordate, margin entire with 1–2 mm long black teeth at the end of the veins (to 5 per side), the leaf, apex acute
to acuminate, coriaceous to frequently chartaceous; adaxial surface often glabrous, sometimes with a few scattered
hairs more dense on the veins, abaxial surface arachnoid (occasionally sparsely pubescent) with long ferruginous
wavy hairs, conspicuously dense on the protruding veins and persistent on the leaf margin; 5–6 main veins arising
from the midrib on each side and numerous conspicuous secondary veins and venules. Inflorescence 10–17 × 1–3.5
cm, narrow, purple; peduncle 3–4.5 cm, slightly hairy; lamellate flowers present.
Distribution.—Indonesia (Java, Sumatra), Malaysia (Peninsula, Sarawak), Thailand (Ranong, Songkhla,
Narathiwat (Fig. 1)).
Ecology.—It grows in evergreen forest. 100–650 m elev. Flowering and fruiting from November to July.
Specimens examined.—INDONESIA. Sumatra: Korthals s.n. (L (L0715762)); Korthals s.n. (L (L0715763),
K). MALAYSIA. East Malaysia: Sarawak, 1865-68, Beccari 796 (FI, FI, FI). Peninsular Malaysia: Pahang,
Phytotaxa 159 (2) © 2014 Magnolia Press • 97
A TAXONOMIC REVISION OF PTERISANTHES IN THAILAND
Gunung Tahan, ca 1,000 m, 19 June 1922, Haniff & Nur 8027 (K); Negri Sembilan, Gumong Angsi falls to west,
610 m, 17 November 1923, Nur 11752 (K); Perak (central), Gopeng Kinta, July 1883, King’s Collector 4621 (K).
THAILAND. Narathiwat: Pah Bukethamong, Ra-ngae district, Sangkachand 917 [BKF no. 54067] (BKF).
Ranong: Khlong Nakha Wildlife Sanctuary, 200–300 m, 2 May 1927, Larsen & Larsen 33585 (AAU, BKF).
Songkhla: Kopah, Ban Krap, Bk. Tinggi, 300 m, 7 November 1917, Haniff & Nur 2728 (SING).
FIGURE 1. Distribution map of P. eriopoda based on specimen data.
Nomenclature notes.—Although originally named as P. o v a t a by Korthals it was never published and
therefore the name is invalid. When Miquel (1863) was writing the account for these species using Korthals’
specimens, he did not recognise Pterisanthes as a genus but a section within Vitis, thus he had to find a new name
for it and named it Vitis eriopoda Miq.
TRIAS-BLASI ET AL.98 • Phytotaxa 159 (2) © 2014 Magnolia Press
In his description of this species Miquel (1863) indicated the presence of a specimen collected by Korthals
from Sumatra. Two specimens with these characteristics were found in L and we believe they are both duplicates of
the type specimen because they were collected by Korthals and the labels indicate the unpublished name given by
this author (P. ovata). We have selected the specimen with barcode L0715762 as a lectotype because it is the most
representative of this species.
In the first description of Pterisanthes coriaceae var. araneosa, King (1896) listed four syntypes, all collected
in Perak. One of them was collected by “King’s collector” (No. 646), while the remaining specimens were
collected by Wray (Nos. 2556, 3615, 4621). A specimen was found in the K herbarium supposedly collected by
“King’s collector” but with one of Wray’s collection number mentioned above (No. 4621). The information on the
label suggests that this specimen was in fact collected by Hermann Kunstler, who collected for King, as the
collection dates, localities and notes match with his known itinerary (Narayanaswami 1933). It is possible the
details on the labels got mixed up at some point as specimens from both collectors were likely sent from the State
Museum in Taiping, where Wray was the Curator, to the Botanical Gardens in Calcutta. Regardless, the specimen
matches King’s description and we have selected it here as the lectotype. This taxon was later recognised as a
synonym of P. eriopoda (Latiff 1982).
The synonym P. beccarina was described by Planchon (1887) from a specimen collected by Beccari in
Sarawak. We have found three isotypes in FI and we have selected the one with FI herbarium number 2743 (not
2743A or 2743B) to be the lectotype.
Latiff (1982) mentioned the synonym P. polita var. lanceolata, which was described by Ridley (1893) from a
specimen collected by Haviland in the Malay Peninsula. Most of Haviland’s collections are in SING and K,
however we have been unable to locate a relevant specimen after extensive searches in these and other herbaria.
Additionally, we have found no specimens labelled with the above name nor have we found specimens with clearly
lanceolate leaves as per Ridley’s (1893) description. Thus, this is a case of incertae sedis as we are unable to
confirm the taxonomic status of this taxon.
Pterisanthes cissioides Blume (1825: 193) [as cissioïdes]. Vitis cissioides (Blume) Backer (1911: 245). Lectotype
(designated here):—INDONESIA. Java, Bogor, Blume s.n. (L! (electronic image with barcode L0747475);
isolectoype L! (electronic image with barcode L0715778).
Vitis ptersiantha Miquel (1863: 94). Holotype:—INDONESIA. Sumatra, Korthals s.n. (L! (electronic image with barcode
L0715787); isotype L! (electronic image with barcode L0715788)).
Ptersianthes dalhousiae var. major Ridley (1922: 481). Holotype:—MALAYSIA. Peninsular Malaysia, Perak, Hermitage,
December 1887, Curtis 1289 (K!).
Pterisanthes trifoliata Merrill (1929: 182). Lectotype (designated here):—MALAYSIA. Sabah, Tawao, Elphinstone Province,
British North Borneo, October 1992 to March 1923, Elmer 20829 (UC!; holotype PNH likely lost; isolectotypes K!, L!
(electronic image with barcode L0013712)).
Tendrils branching from the inflorescence peduncle, mostly simple, 10–15 cm long, slender, glabrous. Leaves
compound, trifoliate or 5-pedate; petiole 3–10 cm long, glabrous; terminal leaflet leaf blade ovate to elliptical, 10–
19 × 4–8 cm, base attenuate, petiolule 2–3.5 cm long; lateral leaflet leaf blade ovate to elliptical, 10–14 × 3–5 cm,
base obliquely rounded, petiolule 0.5–1.5 cm long; margin conspicuously dentate with teeth 0.5–1 mm long at the
end of the secondary veins, apex acute to apiculate; adaxial side glabrous, abaxial arachnoid then becoming
glabrescent; 5–6 main veins growing from the midrib on each side and numerous secondary veins. Inflorescence
10–13 × 3–7 cm, rectangular, brownish-green; peduncle to 26 cm × 1.5 mm, slightly hispid with minute hairs 0.1
mm long; lamellate flowers present; pedicellate flowers pedicel 1.5–2.5 cm long; Fruit reddish, 1–2 seeded,
globose, 0.75 cm diameter. Fig. 2.
Distribution.—Brunei Darussalam, Indonesia (Java, Kalimantan, Sumatra), Malaysia (Peninsula, Sabah),
Thailand (Phangnga, Narathiwat (Fig. 3)).
Ecology.—Forest border, mixed forest, tropical rainforest, thickets. 50–1200 m elev. Flowering from January
to February.
Specimens examined.—BRUNEI DARUSSALAM. Tutong District, Upstream from Belabau on Tutong river,
20 m, 28 March 1990, M.J.E. Coode et al. 6357 (K). INDONESIA. Java: Bogor, Blume s.n. (L (L0715776));
Bogor, Blume s.n. (L (L0715778)); Bogor, Blume s.n. (L (L0747475), (L0715778)). Kalimantan: Wanariset, Plot
Phytotaxa 159 (2) © 2014 Magnolia Press • 99
A TAXONOMIC REVISION OF PTERISANTHES IN THAILAND
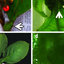
FIGURE 2. Pterisanthes cissioides Blume. Flowering Stem. From Puudjaa 459 (BKF). Drawing by A. Teerawatananon.
TRIAS-BLASI ET AL.100 • Phytotaxa 159 (2) © 2014 Magnolia Press
Matthys, 1° S 117° E, 50 m, 15 May 1990, van Balgooy & P. Kessler 5959 (K); Serawai. Desa Jelundung, 0° 29’
43.5’’ S 112° 32’ 3.1’’ E, 120 m, 2 October 1995, Church, Ismail, Ruskandi 2187 (K); Ketapang. Gunung Palung
National Park, Cabang Panti Research Site, 1° 13’ S 110° 6’ E, 20 m, 24 October 1996, Laman, Rachman,
Mirmanto TL112 (K). Sumatra: Korthals s.n. (L (L0715787), (L0715788)). MALAYSIA. East Malaysia: Sabah,
Tawao, Elphinstone Province, British North Borneo, October 1992 to March 1923, Elmer 20829 (K, L, UC);
Sabah, Mount Kinabalu, Tenompok, 1500 m, 20 February 1932, J. & S. Clemens 26042 (K). Peninsular
Malaysia: Pahang, Sg. Serunai, 17 October 1931, Osman 28218 (K); Perak, Hermitage, December 1887, Curtis
1289 (K); Perak, July 1885, King’s Collector 7914 (K). THAILAND. Narathiwat: Bala-Hala, Waeng, 24 January
1998, Puudjaa 459 (BKF). Phangnga: Sra Nang Manora Forest Park, Muang, 60 m, 23 February 2001,
Chayamarit, Pooma, Chamchumroon 2658 (BKF).
FIGURE 3. Distribution map of P. cissioides based on specimen data.
Phytotaxa 159 (2) © 2014 Magnolia Press • 101
A TAXONOMIC REVISION OF PTERISANTHES IN THAILAND
Nomenclature notes.—In 1825 Blume described the genus Pterisanthes based on a specimen collected in
Java, which he named P. cissioides (at the time P. cissioïdes). We have selected here the lectotype for P. cissioides
from a specimen found in L with a label written in Blume’s handwriting (Van Welzen pers. comm.). Even though it
is quite likely that this specimen is the holotype, we are not completely certain since there are several specimens of
P. cissioides in L with Blume’s handwriting; therefore, we have selected the most representative specimen with the
species name in Blume’s handwriting as the lectotype (barcode L0747475).
In 1863, Miquel described the species Vitis pterisantha from a specimen collected by Korthals in Sumatra; this
has later been recognised as a synonym of P. cissioides. The holotype of this specimen was also thought to be held
in L. Two duplicates from Korthals’ collections with the species name written on the label were found; one of the
duplicates was sent to the herbarium in Groningen (Van Welzen pers. comm.) but after its closure, the specimen
was returned to L. For this reason, the specimen that was kept in L is most likely to have been used as the holotype.
Ridley (1922) described the variety P. dalhousiae var. major from a specimen collected by Curtis in Hermitage
Hill, Perak (Peninsular Malaysia). A specimen with these characteristics was located in the K herbarium and it is
considered to be the holotype as no other duplicates have been found. Latiff (1982) suggested this variety should be
a synonym of P. cissioides and we are in agreement. However, he indicated the wrong type specimen; which was
also collected by Curtis, but in a different locality (Peninsular Malaysia, Penang, West Hill) and it was identified by
Latiff himself as the morphologically distinct P. dalhousiae.
The synonym P. trifoliata was described by Merrill (1929) from a specimen collected by Elmer in Sabah. It is
known that the top set of Elmer’s collections was generally stored in PNH (Van Steenis-Kruseman 1950), but as
Manila was almost completely destroyed in 1945 during World War II, it is quite likely that the holotype was lost.
However, we have found three isotypes in different herbaria; only one of them has an inflorescence present, and
this has been selected as the lectotype.
Pterisanthes polita (Miq.) Lawson (1875: 663). Vitis polita Miquel (1863: 95). P. coriacea Korth. ex King (1896:
693). nom. superfl. Lectotype (designated here):—INDONESIA. Sumatra, in fruticetis prope Pao, Korthals s.n.
(lectotype L! (electronic image with barcode L0013706); isolectotypes L! (electronic image with barcode
L0013704, L0013705, L0013707)).
Pterisanthes sinuosa Merrill (1907: 423). Lectotype (designated here):—PHILIPPINES. Mindanao, Lake Lanao, Camp
Keithley, November 1906, Clemens 647 (A! (electronic image).
Pterisanthes parvifolia Merrill (1917: 76). Lectotype (designated here):—MALAYSIA. Sarawak, Baram District, Marudi, 26
October 1894, Hose 231 (K!; isolectotypes, A! (electronic image with barcode 00051640), BM, L! (barcode L0672034).
Pterisanthes gladiata Van Steenis (Van Steenis & Bakhuizen Van Den Brink 1967: 388) Holoype:—Sabah, Mount Kinabalu,
1200–1500 m, 15 February 1933, J. & M. Clemens 31582 (L! (electronic image with barcode L0013703)).
Tendrils branching from the inflorescence peduncle, mostly simple, sometimes bifurcate, slender or tendrils
unrelated to the inflorescence, simple, robust, leaf-opposed. Leaves simple; petiole 3–6.5 cm long, glabrous; leaf
blade cordate, deltoid to ovate, to 18 × 10.5 cm, base cordate, margin entire with minute teeth at the end of the
venations, apex acute to acuminate, coriaceous to frequently chartaceous, both sides glabrous, shiny with numerous
veins protruding; 6–8 main veins growing from the midrib and numerous secondary veins and venules all
extremely conspicuous. Inflorescence 10–28 × 1–3 cm, narrowly rectangular to gladiate, green turning reddish
when mature; peduncle 5–15 cm long, slender, glabrous; lamellate flowers present; pedicellate flowers, pedicel 1–
2.5 cm long.
Distribution.—Brunei Darussalam, Indonesia (Kalimantan, Sumatra), Malaysia (Peninsula, Sabah, Sarawak),
Myanmar (Peninsula), Philippines (Mindanao), Singapore, Thailand (Peninsula) [specimen lost]. Fig. 4.
Ecology.—Forest border, mixed forest, tropical rainforest. 15–3300 m elev. Flowering and fruiting all year.
Specimens examined.—BRUNEI DARUSSALAM. Tutong, Rambai, Ladan Hill Forest Reserve, Bukit
Bedawan, Southern of LP-263, 4° 29’ 33’’ N, 114° 48’ 52’’ E, 485 m, 26 March 1997, Kalat et al BRUN 18076
(K); Temburong Distr., Subd. Amo. Upper Belalong river west of Bukit Belalong, 4° 30’ N, 115° 08’ E, 130 m, 24
March 1991, Johns et al. 7021 (K); Belait, Melilas, Ulu Sungai Belait, along trail from Ingei to Melilas-Sukang, 4°
10’ N, 114° 42’ E, 25 m, 25 August 1995, Kalat et al. BRUN 17075 (K); Nelait Labi, ukit Teraja, ridge running N
from summit, 4° 18’ N, 114° 26’ E, 350 m, 18 October 1991, Simpson with Marsh 2124 (K). INDONESIA.
Kalimantan: G. Bentuang area, 5–10 km NE of Pontianak, W Kalimantan province, beside Sembawang river, 0°
TRIAS-BLASI ET AL.102 • Phytotaxa 159 (2) © 2014 Magnolia Press
52’ N, 100° 26’ E, 150 m, 8 June 1989, Burley, Tukirin et al. 2351 (K); East Kalimantan, PT. Limbang Ganeca, Ulu
Mahakam, Belayan river area, 0° 12’ N, 116° 02’ E, 50 m, 15 June 1999, Sidiyasa & Ambriansyah 1656 (K).
Sumatra: in fruticetis prope Pao, Korthals s.n. (L (L0013706, L0013704, L0013705, L0013707)); N Sumatra, Bt.
Lawang, Bohorok, Langkat, 200 m, 25 February 1973, Dransfield 3331 (K); old jungle near the Aek Kanopak,
Loendoet concession, Koealoe, 1–17 April 1927, Bartlett 7321 (K). MALAYSIA. East Malaysia: Sabah, Mount
Kinabalu, 1200-1500 m, 15 February 1933, J. & M. Clemens 31582 (L! (electronic image with barcode
L0013703)); Sabah, Tenom district, HS. Kalang, Summit of Kalang hill, 3300 m, 18 September 1991, Gambio et
al. 133697 (K); Sabah, Beluran district, West of Bt. Luminitong, 11 March 1982, Gibot SAN94482 (K); Sabah,
Sandakan district, Sg. Ruku-ruku, Telupid, 6 August 1981, Gibot SAN94034 (K); Sarawak, Baram District,
Marudi, 26 October 1894, Hose 231 (K, A (electronic image with barcode 00051640), L (barcode L0672034);
Sarawak, 1865-68, Beccari 1333 (K); Sarawak, Bkt. Kelaby, Ulu Dapoi, Tinjar, Marudi, 4th division, 122 m, 3 April
1965, Pa’ie S22945 (K); Sarawak, Gunong, Api, ulu Melinau, Tutoh, Baram District, NE flank of mountain, 4° 07’
N 115° 15’ E, 850m, 1 October 1971, Anderson S30870 (K); Sarawak, Gunong Gading, Lundu District, 2st
division, 683 m, 22 September 1974, Mamit S35129 (K). Peninsular Malaysia: Perak, Taiping, March 1894,
Scortechini 111 (BM); Perak, Blanjo?, 30.5 m, L. Wray 150 (K). MYANMAR. Moulmeine, Lobb s.n. (K).
PHILIPPINES. Mindanao: Lake Lanao, Camp Keithley, November 1906, Clemens 647 (A! (electronic image)).
SINGAPORE. Chanchu Kang, November? 1889, N.H.R. s.n. (BM).
Nomenclature notes.—This species was first described as Pterisanthes coriacea by Korthals, however this
was never published and thus the name remained invalid. At the time Miquel (1863) was writing the account for
these species using Korthals’ specimens, he did not recognise Pterisanthes as a genus but a section within Vit i s , so
he had to find a new name for this species. Since the name “Vitis coriacea” was already in use as Cissus coriacea
DC and Vitis coriacea Miq., Miquel (1863) gave the species the new name Vitis polita Miq. Independently, in 1896
King validated the name Pterisanthes coriacea.
A total of four P. polita specimens labelled as isotypes and likely to have been used by Miquel (1863) were
found at L. We have selected the specimen with barcode L0013706 as the lectotype as we believe it is the most
representative for this species. Although this specimen could be the holotype for this species, we cannot be
completely sure since neither the first description, nor the label indicate any distinctive data and therefore a
lectotype status is more suitable here.
Merrill described two new Pterisanthes species (P. sinuosa (1907) and P. parvifolia (1917)), both of which
have since been synonomised under P. polita (Latiff 1982). Pterisanthes sinuosa was described from a specimen
from the Philippines collected by Clemens. It is known that the top set of Clemens’ collections from Mindanao
were generally stored in PNH (Van Steenis-Kruseman 1950), but as Manila was almost completely destroyed in
1945 during World War II, it is quite likely that the holotype was lost. An isotype was found at A and it has been
selected as the lectotype. Pterisanthes parvifolia was described from a specimen from Sarawak (Malaysia)
collected by Hose. Duplicates of the type collection have been found in several herbaria and we have selected here
as the lectotype the one that represents best the taxon.
We have only been able to find one record of a specimen belonging to P. polita occurring in Thailand. This
specimen was reported by Latiff (1982) as being located in K and has the same collection details as another
specimen in SING that Latiff (1982) identified as P. eriopoda in the same publication. We have been able to
confirm the identification of the specimen in SING but it seems that the one held in K has been lost and therefore
we are unable to confirm its taxonomic status. However, records of P. polita have been reported in localities close
to the southernmost Thai provinces in the Malay Peninsula such as the state of Perak. Hence, we think P. polita is
extremely likely to occur in Thailand and therefore it has been included in this account.
Morphological notes.—The species P. polita and P. eriopoda are in fact very similar, with the main difference
being the presence/absence of the pedicel in the flowers, which is the character used by Latiff (1982) to separate
Pterisanthes into two sections. P. polita has pedicellate flowers along the margin of the lamella, while P. eriopoda
does not have pedicellate flowers associated on the lamella. These two species together with P. cissioides are the
most widely distributed and also have the most intraspecific variation within Pterisanthes (Latiff 1982). The
morphological difference used to separate P. polita from P. eriopoda could be explained by this wide range of
variation within species, thus suggesting that they are in fact the same species. However, upon examination of
several specimens we have noticed the following additional differences: 1. Petiole length in P. eriopoda is shorter
(under 3 cm), while P. polita is longer (over 3 cm); 2. Leaves in P. eriopoda have a hairy margin covered with wavy
Phytotaxa 159 (2) © 2014 Magnolia Press • 103
A TAXONOMIC REVISION OF PTERISANTHES IN THAILAND
ferruginous hairs, while leaves in P. polita are glabrous. Due to all these observations we have decided to maintain
them as separate species.
FIGURE 4. Distribution map of P. polita based on specimen data.
Acknowledgements
This work formed part of the Ph.D. thesis of the first author and was supported by a Trinity College Postgraduate
Award, the Trinity College Postgraduate Travel Fund, Synthesys (European Union-Funded Integrated
Infrastructure Initiative Grant), the Davis Expedition Fund, the IAPT Research Grants Program in Plant
TRIAS-BLASI ET AL.104 • Phytotaxa 159 (2) © 2014 Magnolia Press
Systematics, the William Dickson Travelling Fund, the TRF/BIOTEC Special Program for Biodiversity Research
and Training Grant and the Trinity College Dublin Botany Department. The authors are grateful to the staff and
students at the Department of Botany in Trinity College Dublin and especially to Dr. Caroline Byrne. The authors
are also grateful to the staff in A, AAU, BKF, BM, FI, K, L, SING and UC for their help and for the loan of or
access to specimens, in particular to Anthony Brach (A), Julie Shapiro (A), Somran Suddee (BKF), Ranee Prakash
(BM), Egildo Luccioli (FI), Serena Lee (SING), Siti Nur Bazilah Mohamed Ibrahim (SING) and Ana Penny (UC).
Thanks to Prof. Dr. Peter C. Van Welzen for his helpful suggestions during the examination process of the Ph.D.
thesis and for help with L specimens.
References
Agardh, J.G. (1858) Theoria systematis plantarum; accedit familiarum phanerogamarum in series naturales dispositio. C.W.K.
Gleerup, Lund, 404 pp.
Backer, C.A. (1911) Schoolflora voor Java. N.V. Boekh. Visser & Co, Weltevreden, 676 pp.
Blume, C.L. (1825) Ampelideae. In: Blume, C.L. Bijdragen tot de flora van Nederlandsch Indie, 1. Ter Lands Drukkenj,
Batavia, pp. 180–195.
http://dx.doi.org/10.5962/bhl.title.395
Griffith, W. (1854) Notulae ad Plantas Asiaticas, 4. Charles A. Sarrao, Calcutta, 764 pp.
King, G. (1896) Materials for a Flora of the Malayan Peninsula, Disciflorae 6-8. Baptist Mission Press, Calcutta, 802 pp.
Kuntze, O. (1891) Revisio generum plantarum. 1. Arthur Felix: Leipzig, 374 pp.
Latiff, A. (1982) Studies in Malesian Vitaceae I-IV. Federation Museums Journal 27: 46–93.
Latiff, A. (1987) Portraits of threatened plants. 13. Pterisanthes pulchra Ridl. Malayan Naturalist 41(2): 25–26.
Latiff, A. (2001) Diversity of the Vitaceae in the Malay Archipelago. Malayan Nature Journal 55: 29–42.
Lawson, M.A. (1875) Ampelideae. In: Hooker, J.D. (ed.) Flora of British India, 1. L. Reeve & Co., London, pp. 644–668.
Merrill, E.D. (1907) Some genera and species new to the Philippine flora. Philippine Journal of Science. Section C, Botany 2:
421–428.
Merrill, E.D. (1917) Contributions to our knowledge of the Flora of Borneo. Journal of the Straits Branch of the Royal Asiatic
Society 76: 75–117.
Merrill, E.D. (1929) Plantae Elmerianae Borneenses. University of California Publications in Botany 15: 1–316.
Merrill, E.D. (1934) New Sumatran plants, I. Papers of the Michigan Academy of Science, Arts and Letters 19: 149–203.
Miquel, F.A.W. (1863) Annales Musei Botanici Lugduno-Batavi, 1. Van der Post, Amsterdam, 332 pp.
Narayanaswami, V. 1933. Provenance of early Malayan Plant Collections. Journal and Proceedings of the Asiatic Society of
Bengal 27: 327–477.
Planchon, J.E. (1887) Monographie des Ampélidées vrais. In: De candolle, A.F.P.P. & De Candolle, C. (eds.) Monographiae
Phanaerogamarum, 5. G. Masson, Paris, pp. 305–654.
Ridley, H.N. (1893) On the flora of the eastern coast of the Malay Peninsula. Transactions of the Linnean Society of London.
Series 2. 3: 267–408.
http://dx.doi.org/10.1111/j.1095-8339.1893.tb00678.x
Ridley, H.N. (1912) A botanical excursion to Pulau Adang. Journal of the Straits Branch of the Royal Asiatic Society 61: 46.
Ridley, H.N. (1922) Ampelideae. In: Ridley, H.N. The Flora of the Malay Peninsula, 1. L. Reeve & Co. Ltd, London, pp. 469–
487.
Ridley, H.N. (1931) Additions to the Flora of Borneo and Other Malay Islands: III Bulletin of Miscellaneous Information,
Royal Gardens, Kew 1931(10): 493–499.
Soejima, A. & Wen, J. (2006) Phylogenetic analysis of the grape family (Vitaceae) based on three chloroplasts markers.
American Journal of Botany 93: 178–187. DOI: http://dx.doi.org/10.3732/ajb.93.2.278
Trias-Blasi, A., Parnell, J.A.N. & Hodkinson, T.R. (2012) Multi-gene Region Phylogenetic Analysis of the Grape Family
(Vitaceae). Systematic Botany 37: 941–950.
http://dx.doi.org/10.1600/036364412x656437
Van Steenis, C.G.G.J. & Bakhuizen Van Den Brink, R.C. (1967) Miscellaneous botanical notes XVI. 104: Two new Bornean
Pterisanthes (Vitaceae). Engler's Botanische Jahrbücher 86: 385–390.
Van Steenis-Kruseman, M.J. (1950) Malaysian Plant Collectors and Collections: Being a Cyclopaedia of Botanical Exploration
in Malaysia and a Guide to the Concerned Literature to the Year 1950. In: Van Steenis, C.G.G.J. Flora Malesiana, Series 1,
Volume 1. Noordhoff-Kolff, Jakarta, 1–639 pp.
Wen, J. (2007) Vitaceae. In: Kubitzki, K. The families and genera of vascular plants, Vol.9: Flowering plants. Eudicots:
Berberidopsidales, Buxales, Crossosomatales, Fabales p.p., Geraniales, Gunnerales, Myrtales p.p., Proteales,
Saxifragales, Vitales, Zygophyllales, Clusiaceae Alliance, Passifloraceae Alliance, Dilleniaceae, Huaceae,
Picramniaceae, Sabiaceae. Springer-Verlag, Berlin, 467–479 pp.
Wen, J., Nie, Z.-L., Soejima, A. & Meng, Y. (2007) Phylogeny of Vitaceae based on the nuclear GAI1 gene sequences.
Canadian Journal of Botany 85: 731–745.