Content uploaded by César A. C. Sequeira
Author content
All content in this area was uploaded by César A. C. Sequeira on Aug 18, 2014
Content may be subject to copyright.
D. M. F. Santosa,*, B. Šljukića, L. Amarala, D. Macciòb, A. Sacconeb,
and C. A. C. Sequeiraa
1
a Materials Electrochemistry Group, ICEMS, Instituto Superior Técnico, University of Lisbon,
Av. Rovisco Pais, 1049–001 Lisboa, Portugal
b Università di Genova, Dipartimento di Chimica e Chimica Industriale, via Dodecaneso 31, I-16146 Genova, Italy
* E-mail: diogosantos@ist.utl.pt
224th ECS Meeting, San Francisco, CA, Oct. 27 –Nov 1, 2013
2
Sodium borohydride (NaBH4) as a fuel
Na
H
H
H
H
B
stable in alkaline media
readily available
gives water-soluble discharge products
carries a large amount of hydrogen (10.6 wt. %) in a safe and compact form
no carbon dioxide is produced during its oxidation in FCs
environmentally accepted oxidation product, sodium metaborate, with possibility of
recycling to NaBH4
3
Direct Borohydride Fuel Cells (DBFCs)
high theoretical specific
capacity, i.e., 5.7 Ah g-1
(based on NaBH4),
Type of cell DBFC DBPFC
Hydrogen FC
DMFC
Cell voltage / V 1.64 2.11 –3.02 1.24 1.19
Specific energy /
Whg-1 9.25 17.00 3.40 6.09
Anode: BH4
-+ 8 OH-→ BO2
-+ 6 H2O + 8 e-E0 = -1.24 V
Cathode: 2 O2+ 4 H2O + 8 e-→ 8 OH-E0 = 0.40 V
Overall: BH4
-+ 2 O2→ BO2
-+ 2 H2O E0 = 1.64 V
Cathode: H2O2+ 2 e-→ 2 OH-E0 = 0.87 V
Overall: BH4
-+ 4 H2O2→ BO2
-+ 6 H2O E0 = 2.11V
Electrocatalysts for BOR
4
Pt –high kinetics of BH4-oxidation
gold (Au) - the highest coulombic efficiency for BH4-oxidation
palladium (Pd)
silver (Ag)
alloys such as Pt-Au, Pt-Ag, Pt-ruthenium (Ru), Pt-rare earth (RE), Au-cobalt
(Co), rhodium-iridium (Rh-Ir) and osmium (Os) alloys
High cost, low reserves!
Non-noble metals:
nickel (Ni)
cobalt (Co) and its oxide CoO
zinc (Zn)
hydrogen storage AB5and AB2-type
Solution?
Bimetallic Ni alloys (with Rare Earth metals)
•high BOR rates
•increased coulombic efficiency, i.e., higher number of electrons transferred
NaBH4+ x OH-→NaBO2+ (x - 2) H2O + (4 - x/2) H2+ x e-
5
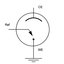
Experimental
One compartment glass cell with three-electrode set up
•Pt mesh (100 cm2)as counter electrode
•Saturated Calomel Electrode (SCE) as reference electrode
•Ni or NiCe alloy as working electrode
0.03 M NaBH4 in 2 M NaOH supporting electrolyte
Temperature controlled in the range from 25 to 45⁰C
Cyclic voltammetry (CV) measurements with scan rates in the range 0.5-3500 mVs-1
Chronoamperometry (CA) studies with different potentials (-0.9, -0.8, -0.7 and -0.6 V)
Fuel cell testing Ni-based electrode | NaBH4, NaOH|| HCl, H2O2|Pt
6
Electrocatalysts characterisation
A - Ni-Ce with 95 at.% Ni: two phase alloy constituted by Ni black crystals (dendritic
shape) and eutectic formed by (Ni+CeNi5)
B - Ni-Ce with 90 at.% Ni: two phases consisting of CeNi5white crystals and eutectic
formed by (Ni+CeNi5). The average eutectic composition is around 90-91 at.% Ni.
Backscattered electron images of the Ni-Ce alloys: A) Ni95Ce5; B) Ni90Ce10.
7
CVs of Ni and Ni-Ce alloy electrodes in 0.03 M NaBH4solution
recorded at a scan rate of 50 mVs-1 and temperature of 25 ºC.
CV study of BOR at Ni and Ni-Ce electrodes
Ni + 2 OH-→α-Ni(OH)2+ 2 e-
Ni + H2O →NiO + 2 H++ 2 e-
Ni(OH)2+ OH-→NiOOH + H2O + e-
BOR
8
CV study of BOR at Ni and Ni-Ce electrodes
- reaction mechanism -
Two mechanisms have been proposed for BH4-oxidation at Ni and Ni-based electrodes.
Stepwise pathway: BHy-dissociation via four steps (y = 4, 3, 2, 1) to H atom and BHy-1-ion.
The generated ions combine with OH-forming BHy-1(OH)-with release of 1 electron in
each step (Eqs. 1 and 2).
BHy(OH)4-y-→BHy-1(OH)4-y-+ H (y = 4, 3, 2, 1) [1]
BHy-1(OH)4-y-+OH-→BHy-1(OH)5-y-+ e-[2]
Second proposed mechanism: rates of the simultaneous BH4-oxidation and hydrolysis
are determined by the potential, as well as the chemical state of electrode surface
(Eqs. 3 and 4).
BHy(OH)4-y-+ 2 OH-→BHy-1(OH)5-y-+ H2O + 2 e-[3]
BHy(OH)4-y-+ H2O→BHy-1(OH)5-y-+ H2[4]
9
CV study of BOR at Ni and Ni-Ce electrodes
- charge transfer coefficient -
1/2
1/2 a
0
p
as
RT D (1 )n F
E E 0.78 ln ln
(1 )n F k RT
Electrode α
Ni 0.69
Ni95Ce50.65
Ni90Ce10 0.53
Dependence of peak potential, Ep, on logarithm of scan rate
for Ni95Ce5alloy electrode in 0.03 M NaBH4solution at 45 ºC.
10
Number of
electrons exchanged
(n) in the BOR at
three studied
electrodes close to 1
Why number of
electrons exchanged
lower than
expected?
CV study of BOR at Ni and Ni-Ce electrodes
- coulombic efficiency -
1/2
5 1/2 1/2
pa
j = 2.99×10 1-α n nCD
Dependence of peak current density, jp, on square root of scan rate, ν1/2,
for Ni95Ce5alloy electrode in 0.03 M NaBH4solution at 45 ºC.
Randles-Sevcik equation
for irreversible processes
11
CV study of BOR at Ni and Ni-Ce electrodes
- influence of temperature -
Anodic scans of Ni95Ce5electrode in 0.03 M NaBH4at a scan rate of 250 mVs-1
for temperatures of 25, 35 and 45 ºC
12
CV study of BOR at Ni and Ni-Ce electrodes
- heterogeneous rate constant -
a0
p s p
(1-α)n F
j = 0.227nFCk exp E -E
RT
Electrode ks
Ni 10-3 cm s-1 order of magnitude
independent of temperature
Ni95Ce510-6 to 10-4 cm s-1 order of magnitude
depending on temperature
Ni90Ce10 10-4 to 10-3 cm s-1 order of magnitude
depending on temperature
13
Chronoamperometry curves of the three studied electrodes recorded in
0.03 M NaBH4solution at potential of -0.9 V and temperature of 45 ºC
CA study of BOR at Ni and Ni-Ce electrodes
Ce + 2 OH-→Ce(OH)22+ + 4 e-
Ce(OH)22+ + 2 OH-→Ce(OH)4
Ce(OH)4→CeO2+ 2 H2O
14
Effect of the operation temperature on the polarization and power density
curves of a DBPFC employing Ni95Ce5anode.
Fuel cell testing
15
25 ºC
35 ºC
45 ºC
Peak power density / mW cm-2 58.1 73.2 110
Cell voltage at peak power density / V 0.45 0.55 0.50
Current density at peak power density / mAcm-2 129 133 219
Effect of temperature on Ni95Ce5| NaBH4, NaOH|| HCl, H2O2| Pt fuel cell.
Fuel cell testing
16
Conclusions
Ni and Ni-Ce alloys of different compositions were prepared by arc melting procedure
and characterized by SEM/EPMA and XRPD.
A thorough CV and CA study of BOR on three Ni-based electrodes, pure Ni, Ni95Ce5and
Ni90Ce50 alloy, has been performed.
Oxidation peak current densities obtained were of similar values at all studied
electrodes, with Ni95Ce5alloy electrode giving the highest current densities.
Kinetic parameters (charge transfer coefficient and heterogeneous rate constant) of
BOR were evaluated several different temperatures.
n values were found to be lower than expected.
Peak power density of 110 mW cm-2 was achieved at a cell voltage of 0.50 V and a
current density of 219 mAcm-2 at 45 ºC in a DBPFC employing Ni95Ce5alloy anode.
17
THANK YOU
FOR YOUR ATTENTION
Acknowledgements
Fundação para a Ciência e a Tecnologia (FCT, Portugal)
Postdoctoral research grants no. SFRH/BPD/91853/2012 (D.M.F. Santos) and
SFRH/BPD/77768/2011 (B.Šljukić)
Research grant within the project PTDC/SEN-ENR/121265/2010 (L. Amaral)